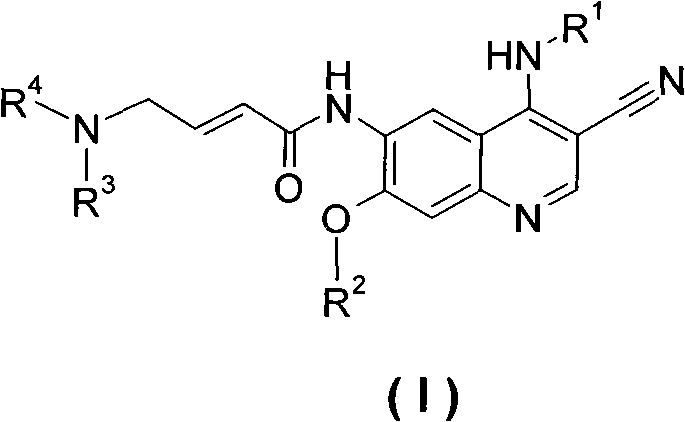
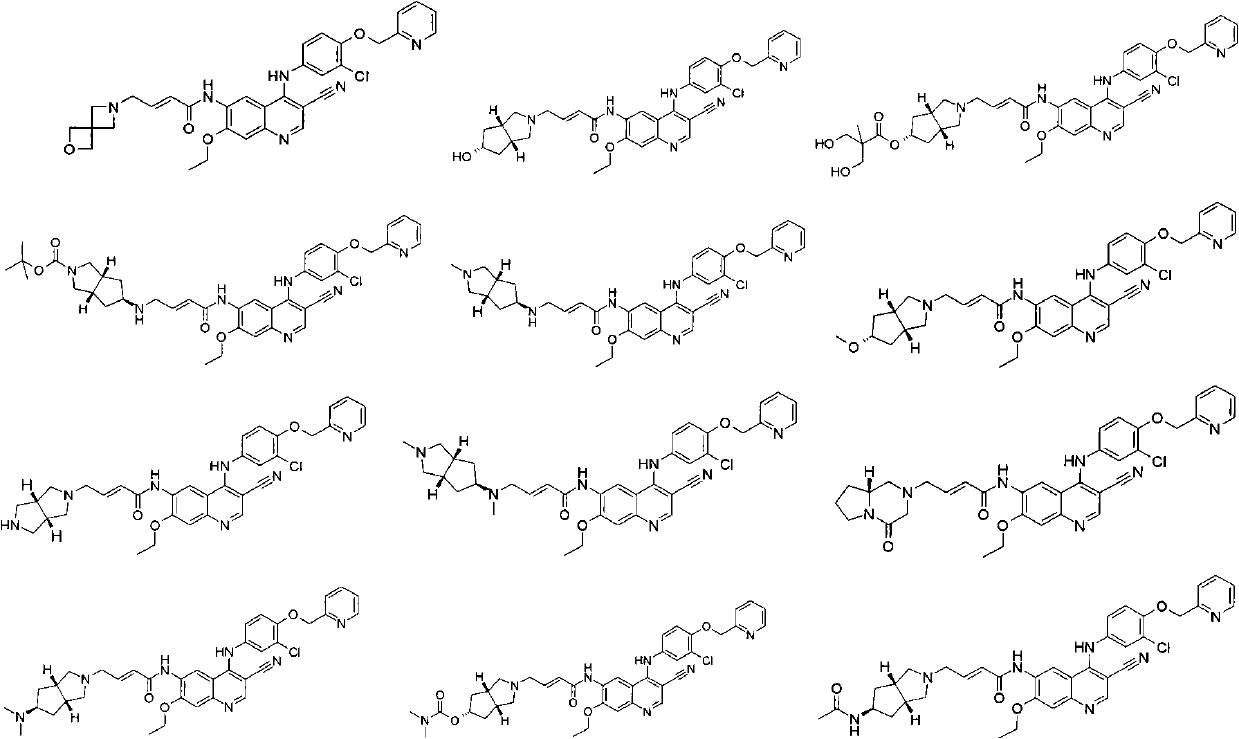
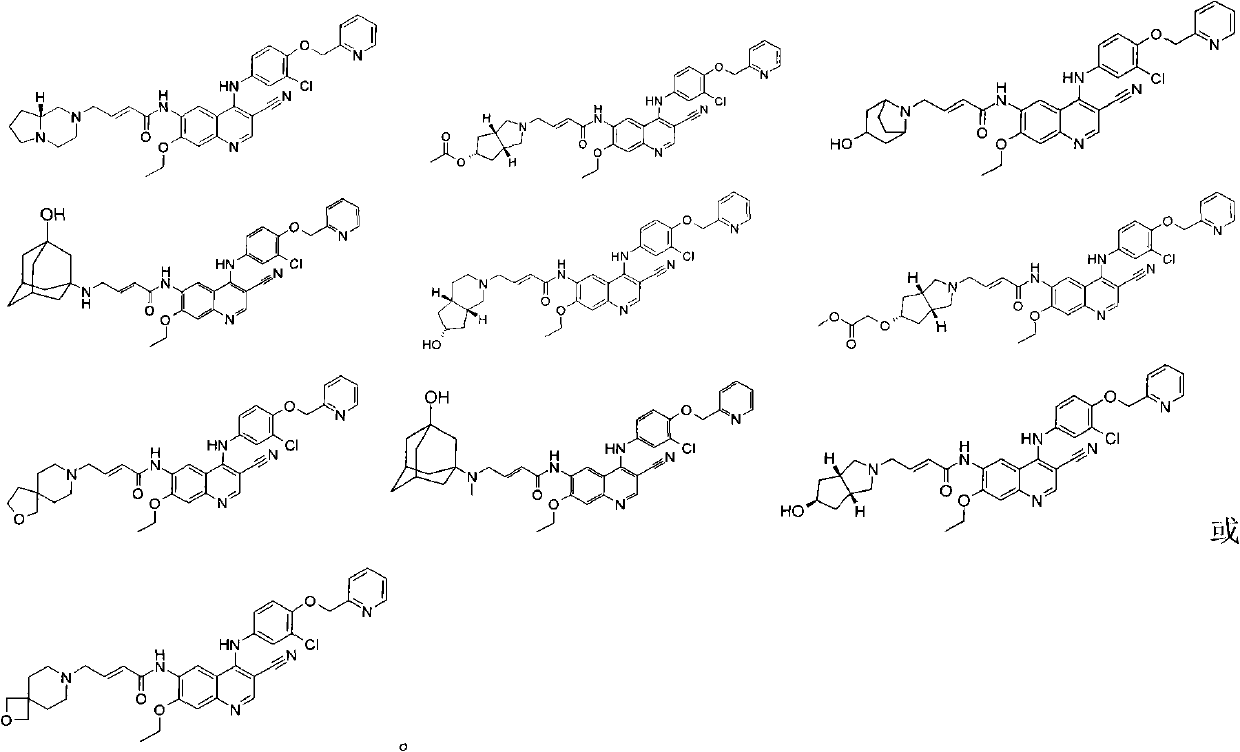
3-cyan-6-aminoquinoline derivatives, preparation method thereof and application thereof in medicines
An alkyl and aryl technology, applied in 3-cyano-6-aminoquinoline derivatives, its preparation and its application in medicine, can solve the problems of increased cell division rate and genome instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] (E)-4-(2-Oxa-6-aza-spiro[3.3]hept-6-yl)-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-yl Methoxy)-aniline Base-3-cyano-7-ethoxy-quinolin-6-yl}-amide
[0137]
[0138] (E)-4-Bromo-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-anilino]-3-cyano-7-ethoxy -Quinolin-6-yl}-amide 1a (396mg, 0.70mmol, prepared by the known method "Journal of Medicinal Chemistry, 2000, 43(17), 3244-3256"), sodium iodide (315mg, 2.10mmol ) and N, N-diisopropylethylamine (0.6mL, 3.50mmol) were dissolved in 5mL N, N-dimethylformamide, and 2-oxa-6-aza-spiro[3.3]heptane was added Oxalate 1b (264 mg, 1.4 mmol, prepared by the known method "Organic Letters, 2008, 10(16), 3525-3526") was reacted with stirring for 12 hours. Pour the reaction solution into 50 mL of ice water, extract with dichloromethane (75 mL×3), combine the organic phases, wash with saturated brine (40 mL×2), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure on silica gel The r...
Embodiment 2
[0142] (E)-4-((3aR, 6aS)-5-hydroxy-hexahydro-cyclopenta[c]pyrrol-2-yl)-but-2-enoic acid {4-[3-chloro-4-( Pyridin-2-yl Methoxy)-anilino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide
[0143]
[0144] first step
[0145] (3aR,6aS)-tert-butyl 5-hydroxy-hexahydro-cyclopenta[c]pyrrole-2-carboxylate
[0146]Dissolve (3aR, 6aS)-5-oxo-hexahydro-cyclopenta[c]pyrrole-2-carboxylate tert-butyl ester 2a (500 mg, 2.22 mmol, prepared according to existing document WO2008089636) in 100 mL methanol , cooled to 0° C. in an ice bath, slowly added sodium borohydride (168 mg, 4.44 mmol), and stirred for 30 minutes. 1 mL of water was added, the reaction solution was concentrated under reduced pressure, extracted with ethyl acetate (50 mL×3), the organic phases were combined, washed with saturated brine (25 mL×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain Crude product (3aR, 6aS)-tert-butyl 5-hydroxy-hexahydro-cyclopenta[c]py...
Embodiment 3
[0156] (3aR, 6aS, E)-3-Hydroxy-2-hydroxymethyl-2-methyl-propionic acid 2-(3-{4-[3-chloro-4-(pyridin-2-ylmethoxy) -aniline Base]-3-cyano-7-ethoxy-quinolin-6-ylcarbamoyl}-allyl)-octahydro-cyclopenta[c]pyrrol-5-yl ester
[0157]
[0158] first step
[0159] 2,2,5-Trimethyl-[1,3]dioxane-5-carboxylic acid
[0160] 3-Hydroxy-2-hydroxymethyl-2-methyl-propionic acid 3a (10.01g, 0.075mol, purchased from Shaoyuan Chemical Technology Co., Ltd.), 2,2-dimethoxy-propane (11mL, 0.09 mol) and p-toluenesulfonic acid monohydrate (1.40 g, 0.075 mol) were dissolved in 100 mL of acetone, and stirred for 12 hours. Concentrate under reduced pressure, dissolve the residue in 100 mL of dichloromethane, wash with water (50 mL×2) and saturated brine (50 mL×2) successively, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain 2, 2, 5 - Trimethyl-[1,3]dioxane-5-carboxylic acid 3b (3.9 g, white solid), yield: 30.0%.
[0161] MS m / z(ESI): 173.1[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com