Deuterated benzylbenzene derivatives and methods of use
一种化合物、基团的技术,应用在治疗受SGLT抑制作用影响的疾病和病症领域,能够解决有害副作用、不能提供充分血糖控制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
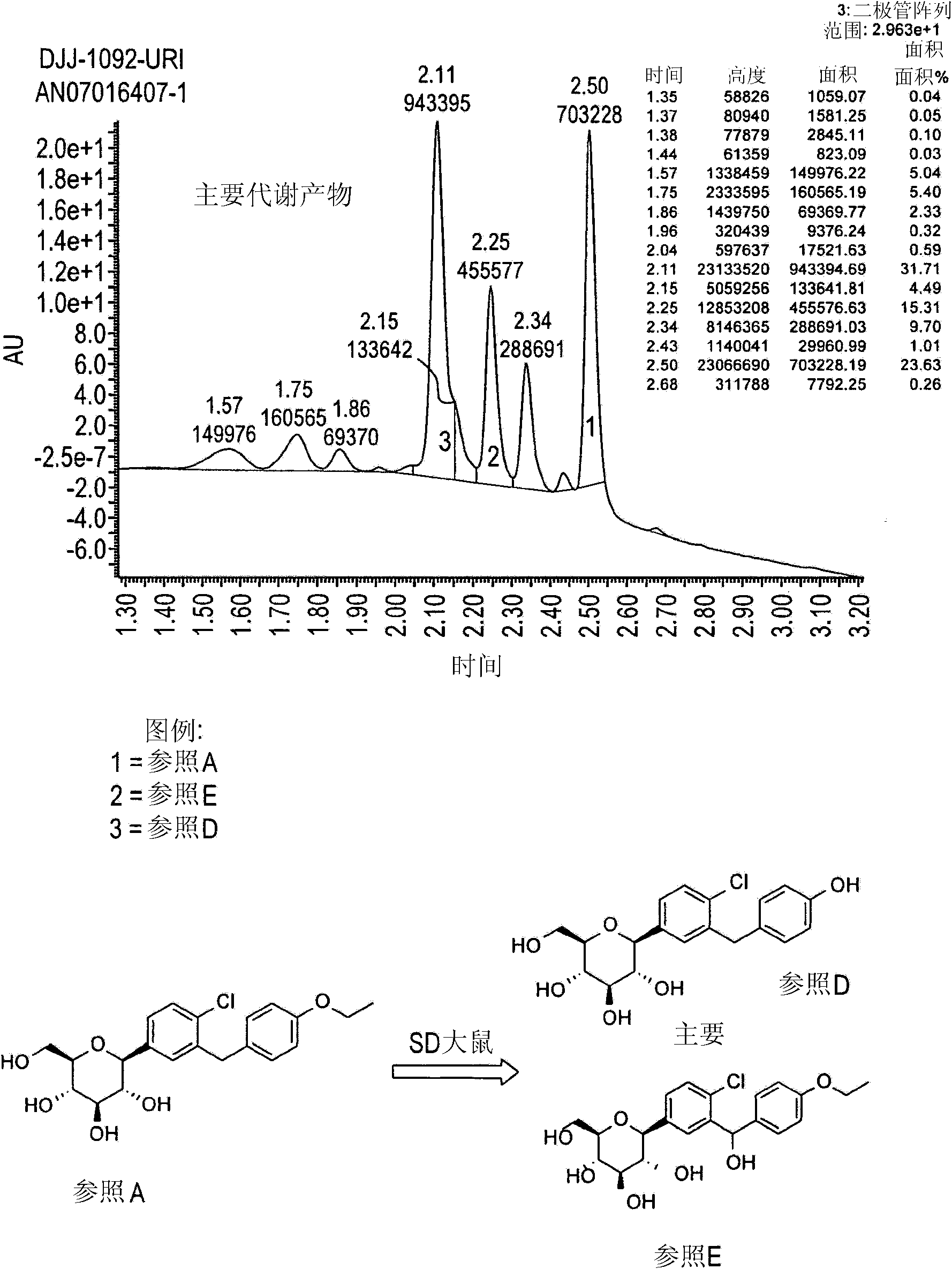
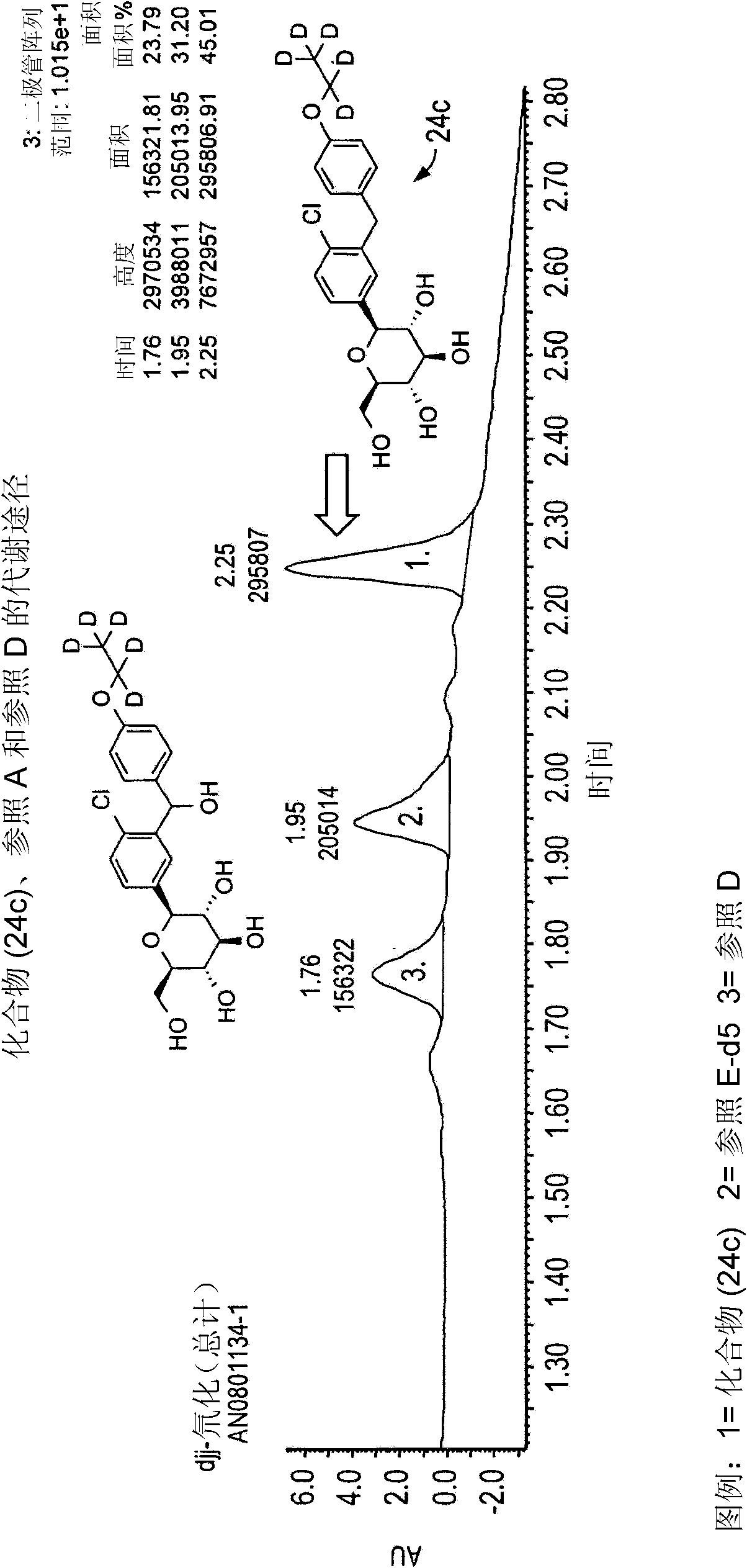
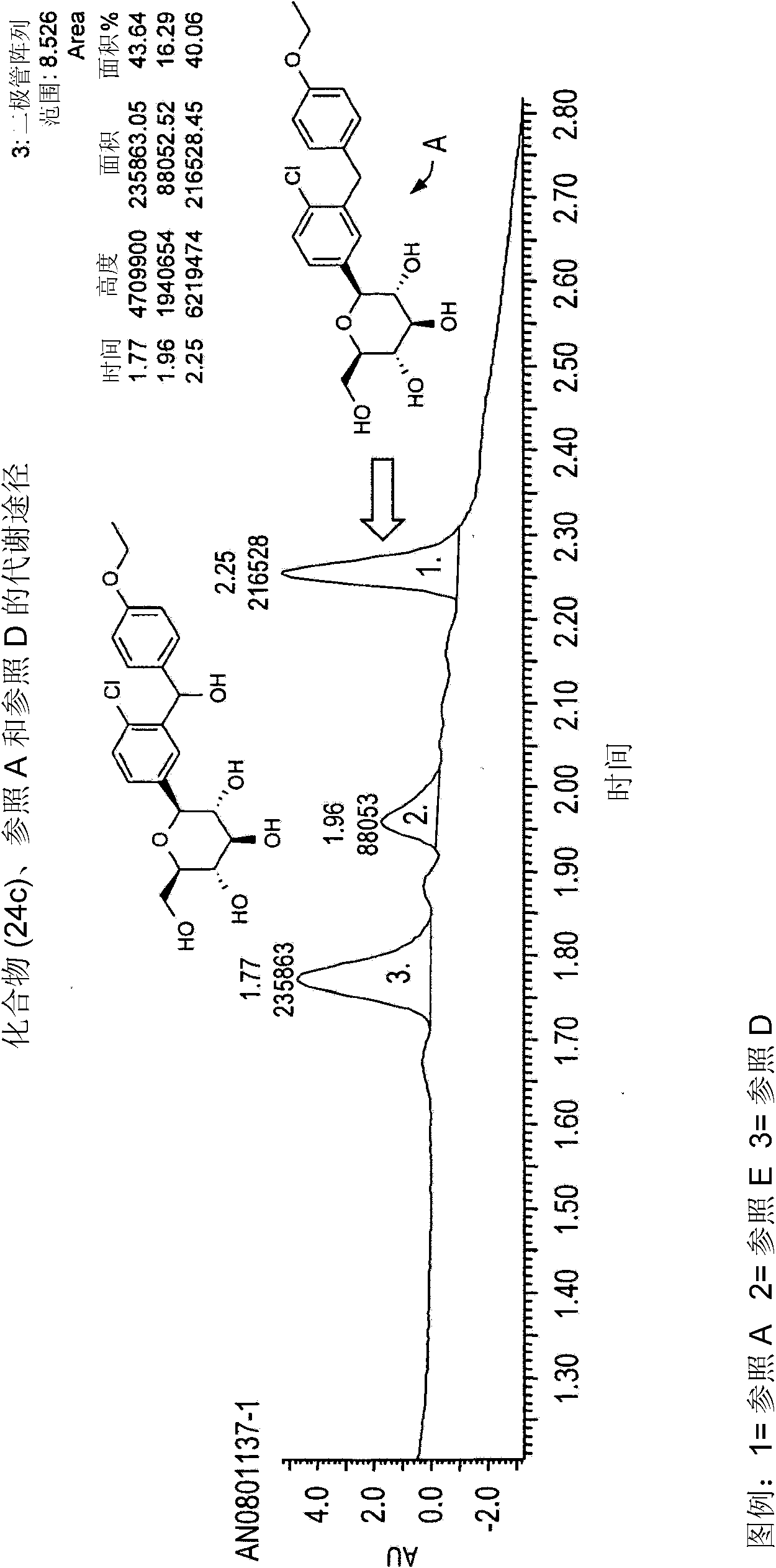
Image
Examples
Embodiment approach
[0199]
[0200] where R 3 independently represents hydrogen, deuterium, halogen, or C 1-C 6 Alkyl; R 8 Independently represent hydroxyl, cyano, C 1 -C 6 Alkyl, C 2 -C 6 Alkenyl, C 2 -C 6 Alkynyl, C 3 -C 10 Cycloalkyl, C 1 -C 6 Alkoxy, C 3 -C 10 Cycloalkoxy, or (C 3 -C 10 Cycloalkyl) C 1 -C 3 Alkoxy, (C 3 -C 7 Cycloalkyl) C 3 -C 5 Alkenyloxy, or (C 3 -C 7 Cycloalkyl) C 3 -C 5 Alkynyloxy; and R 11 , R 12 and R 13 each independently represents hydrogen or deuterium;
[0201] where at least (i) R 11 , R 12 and R 13 one of which is deuterium, or (ii) R 8 from C 1 -C 6 Alkyl, C 2 -C 6 Alkenyl, C 2 -C 6 Alkynyl, C 3 -C 10 Cycloalkyl, C 1 -C 6 Alkoxy, C 3 -C 10 Cycloalkoxy, and (C 3 -C 10 Cycloalkyl) C 1 -C 3 Alkoxy, (C 3 -C 7 Cycloalkyl) C 3 -C 5 Alkenyloxy and (C 3 -C 7 Cycloalkyl) C 3 -C 5 Alkynyloxy, wherein an alkyl, alkenyl, alkynyl, or cycloalkyl, or cycloalkenyl group or moiety is partially or fully substituted with de...
Embodiment 1
[0438] This example illustrates the preparation of Compound 5 according to the method provided in Scheme 9. The general method can be applied to other compounds of the invention.
[0439] Diagram 9
[0440]
[0441] Preparation of (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone (2)
[0442]
[0443] To 5-bromo-2-chlorobenzoic acid (1500 g, 6.41 mol) and oxalyl chloride (975 g, 7.69 mol) in DCM (2.8 L) was added DMF (9 mL). Once the vigorous gas evolution ceased, the reaction was stirred at room temperature for 10 hours. The solution was concentrated under vacuum to obtain a yellow residue. The residue was dissolved in DCM (1.2 L), then the stirred mixture was cooled to -3 °C and phenetole (799 g, 6.54 mol) was added. After the addition was complete, aluminum(III) chloride (973 g, 6.54 mol) was added over 1 hour while keeping the reaction temperature below 4°C. The mixture was poured onto 10 kg of ice and stirred at 4°C for 1 hour. The suspension was diluted with ...
Embodiment 2
[0454] (2S, 3R, 4R, 5S, 6R)-2-(4-chloro-3-(4-ethylbenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-2- Preparation of d-3,4,5-triol (6)
[0455]
[0456] Compound 6 was prepared using a method similar to that described in Example 1 above, using ethylbenzene instead of phenetole as the starting material. 1 H-NMR (CD3 OD, 400MHz): δ736~7.25(m, 3H), 7.08(s, 4H), 4.11~3.99(dd, J=20.4 and 12Hz, 2H), 3.89~3.84(dd, J=11.7 and 1.8Hz, 1H ), 3.70~3.65(m, 1H), 3.45~3.37(m, 3H), 3.27(m, 1H), 2.65~2.55(q, J=15 and 7.5Hz, 2H), 1.21~1.16(t, J =7.5Hz, 3H); MS ESI (m / z): 394 [M+H] + , the calculated value is 393.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com