Chimeric virus of complete structural protein of hepatitis C virus and GB virus B
A hepatitis C virus and structural protein technology, applied to the composition and its preparation or purification, to introduce foreign genetic material modified virus, virus field, can solve the problem that the interaction between the hepatitis C virus and the host cannot be shown, and cannot be completely simulated HCV immune response, inability to fully simulate HCV infection and immune status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
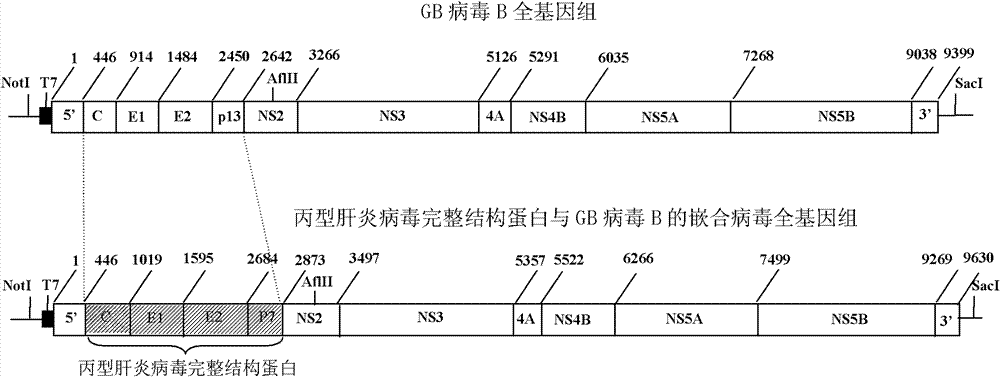
[0033] Embodiment 1, prepare the chimeric virus of hepatitis C virus complete structural protein and GB virus B
[0034] (1) Preparation of the complete structural protein gene of hepatitis C virus
[0035] (a) The viral RNA in the serum sample of hepatitis C virus type 1b was extracted from 200 μl of serum with the Roche nucleic acid extraction kit according to the instructions, and dissolved in 50 μl of Elution buffer.
[0036] (b) Design and synthesize the upstream outer primers, downstream outer primers, upstream inner primers and downstream inner primers for amplifying the complete structural protein gene of hepatitis C virus type 1b; reverse the extracted hepatitis C virus RNA by RT-PCR It was recorded as cDNA; the complete structural protein gene fragment of hepatitis C virus was amplified in two rounds by nested PCR. The amplified complete structural protein gene of the hepatitis C virus is connected with the pMD-20T vector, transformed with TOP10 competent cells, and...
Embodiment 2
[0075] Example 2. Infectivity evaluation of chimeric virus in marmoset
[0076] Evaluation criteria for chimeric virus infectivity: ① Infectious: virus replicates, and the viral load is detected in marmoset serum and verified to be correct; ② Non-infectious: virus does not replicate, and viral load cannot be detected in marmoset serum.
[0077] The method of infecting marmosets can be intrahepatic injection of chimeric virus and intravenous injection of primary marmoset serum containing chimeric virus to infect marmosets. The specific infection steps and evaluation are as follows:
[0078] 1. Intrahepatic injection of chimeric viral RNA and determination of viral load in marmoset serum
[0079] (1) Intrahepatic injection of chimeric virus RNA
[0080]Choose healthy adult marmosets (with normal liver enzyme activity such as ALT, AST, and GB virus B negative) (about 300-400g / monkey), divide them into three groups, expose the liver by surgery, and inject the prepared chimeric v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com