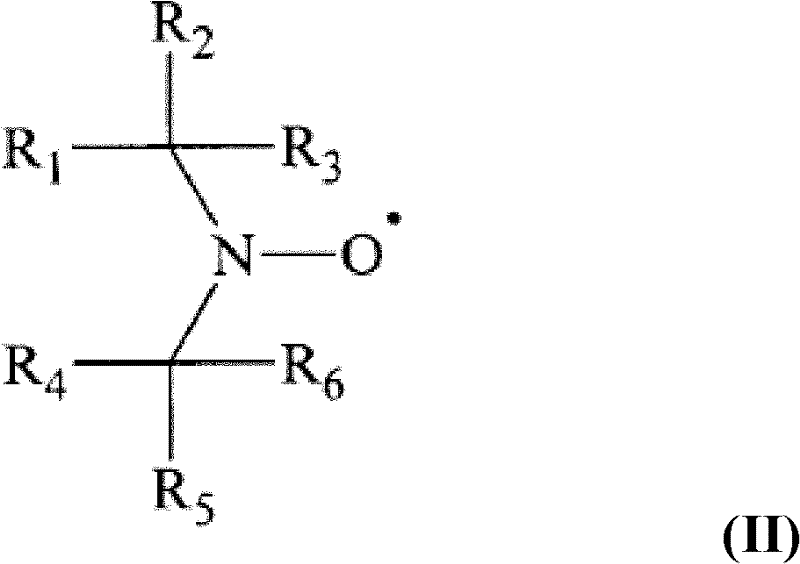
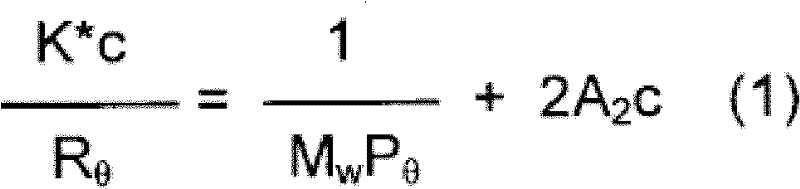
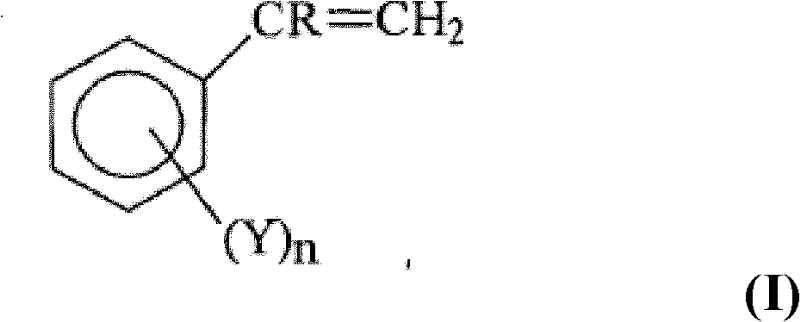Process for the synthesis of functionalized poly(1,3-alkadienes) and use thereof in the preparation of high impact vinyl aromatic polymers
A vinyl aromatic, functionalized technology for the preparation of styrene polymers grafted on functionalized polybutadiene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1 (contrast)
[0102] In a nitrogen stream, the following products were successively introduced into a 100-liter water-free reactor, which was equipped with a stirrer and a heating jacket, and heat transfer oil at a temperature of 50° C. was circulated in the jacket: 50 kg of anhydrous Aqueous cyclohexane, 6.5 kg of anhydrous butadiene without inhibitors and alkynes and 5 g THF. When the reaction mixture reached a temperature of 40° C., 2.6 g of a 15% by weight solution of butyllithium dissolved in cyclohexane was added. When the conversion was complete, a 2.77 g portion of trimethylchlorosilane was fed into the reactor at a temperature of 105°C to achieve complete termination of the chain ends.
[0103] The reagent mixture is then released into a pressurized container where the 565 and 168, added in amounts such that their contents in the elastomer were equal to 0.1 and 0.4%, respectively.
[0104] The polymer is then separated from the solvent by stri...
Embodiment 2
[0110] In a nitrogen stream, the following products were successively introduced into a 100-liter water-free reactor, which was equipped with a stirrer and a heating jacket, and heat transfer oil at a temperature of 50° C. was circulated in the jacket: 50 kg of anhydrous Aqueous cyclohexane, 6.5 kg of anhydrous butadiene without inhibitors and alkynes and 5 g THF. When the reaction mixture reached a temperature of 40° C., 2.16 g of a 15% by weight solution of butyllithium dissolved in cyclohexane was added. When the conversion is complete, a portion of 0.83 g of a 5% solution of butyllithium dissolved in cyclohexane is fed into the reactor at a temperature of 105° C., and after waiting 5′, a second portion of 6.6 g 5% solution of butyllithium dissolved in cyclohexane. Immediately after the second addition of butyllithium, 20 g of octyl bromide in 20% cyclohexane was added, followed immediately by 25.6 g of 1,1,3,3-tetraethylisoindolin-2-yl Oxygen (TEDIO).
[0111] The reage...
Embodiment 3
[0116] Embodiment 3 (contrast)
[0117] In a nitrogen stream, the following products were successively introduced into a 100-liter water-free reactor, which was equipped with a stirrer and a heating jacket, and heat transfer oil at a temperature of 50° C. was circulated in the jacket: 50 kg of anhydrous Aqueous cyclohexane, 6.5 kg of anhydrous butadiene without inhibitors and alkynes and 5 g THF. When the reaction mixture reached a temperature of 40° C., 1.5 g of a 15% by weight solution of butyllithium dissolved in cyclohexane was added. When the conversion was complete, a 2 g portion of trimethylchlorosilane was fed into the reactor at a temperature of 102°C to achieve complete termination of the chain ends.
[0118] The reagent mixture is then released into a pressurized container where the 565 and 168, added in amounts such that their contents in the elastomer were equal to 0.1 and 0.4%, respectively.
[0119] The polymer is then separated from the solvent by strippi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com