Method for extracting and purifying tanshinone monomeric compounds from red sage root
An extract, tanshinone technology, applied in the direction of steroids, quinone separation/purification, organic chemistry, etc., can solve the problems of troublesome operation, low purity of components, etc., and achieves reagent saving, simple method operation, and low content of polar impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0033] Embodiment 1-5: Method for extracting and purifying tanshinone monomer compounds from salvia miltiorrhiza
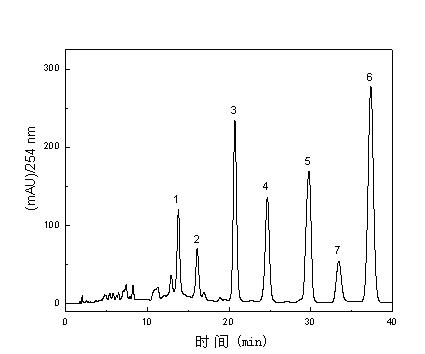
[0034] (1) Preparation of the extract: Take 100 g of crushed Danshen medicinal material (through a 25-mesh sieve), add 500 ml of dichloromethane (soaked for 120 min) and reflux extraction three times, the extraction temperature is 40 °C, and the extraction time is 30 h each time . The three extracts were combined, the dichloromethane was recovered, and the Danshen extract was obtained. figure 2 is the high performance liquid chromatogram of salvia miltiorrhiza dichloromethane extract, by figure 2 It can be seen that the main component of the extract is tanshinone compounds, and the amount of impurities is low.
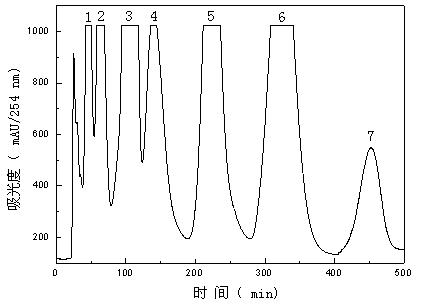
[0035](2) Purification by high-speed countercurrent chromatography: the extract of Salvia miltiorrhiza was dissolved in the lower phase of petroleum ether-n-butanol-methanol-water system as the sample solution for subsequent separation and purificati...
Embodiment 6
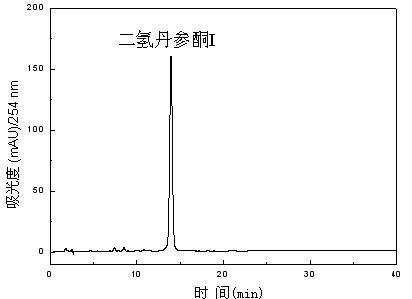
[0050] Example 6 The difference from Examples 1-5 is that in the preparation of the extract in step (1), percolation extraction is used, and a double-layer percolation column with a jacket for heating and heat preservation is used. The temperature of the return medium is 43°C, and the percolation The flow rate was 3 column volumes / hour. According to the high performance liquid chromatogram of each compound, calculate through chromatographic area normalization method, the purity of dihydrotanshinone I (compound 1) is 96.6%, 1,2,15,16-tetrahydrodanshinone (compound 2) The purity of tanshinone (compound 3) is 96.3%, the purity of tanshinone I (compound 4) is 99.3%, the purity of ganxixinone (compound 5) is 96.5%, and the purity of tanshinone IIA (compound 5) is 99.4%. 6) was 99.6% pure, and tanshinone (compound 7) was 99.3% pure.
Embodiment 7
[0051] Example 7The difference from Examples 1-5 is that in the preparation of the extract in step (1), ultrasonic extraction is used, the ultrasonic frequency used is 28KHZ, the extraction time is 40 minutes, and the number of extractions is 2 times. According to the high performance liquid chromatogram of each compound, calculate through chromatographic area normalization method, the purity of dihydrotanshinone I (compound 1) is 96.2%, 1,2,15,16-tetrahydrodanshinone (compound 2) The purity of tanshinone is 96.4%, the purity of cryptotanshinone (compound 3) is 96.4%, the purity of tanshinone I (compound 4) is 96.7%, the purity of ganxixinone (compound 5) is 98.4%, and the purity of tanshinone IIA (compound 6) was 98.6% pure, and tanshinone (compound 7) was 96.3% pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com