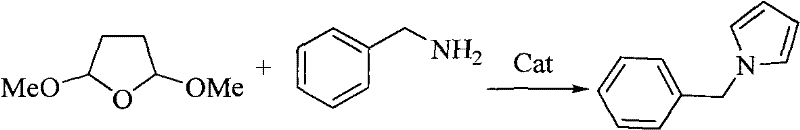
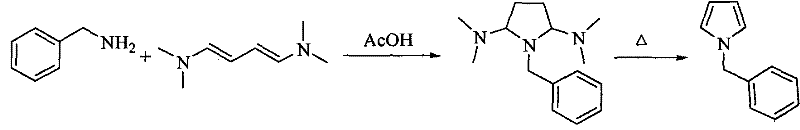
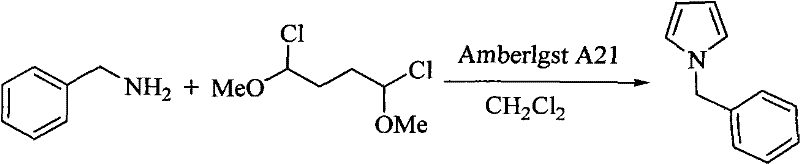
Method for preparing pyrrole derivative
A compound and said technology, applied in the field of preparing pyrrole derivatives, can solve problems such as high price, unstable raw materials such as pyrrole and 3-pyrroline, and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In the preparation method of the present invention, thin layer chromatography (TLC) can be used to judge the end point of the preparation reaction (petroleum ether / ethyl acetate=10:1 (v / v)); and the obtained formula I The crude product of the shown compound can be purified by existing conventional purification methods such as recrystallization or column chromatography (column chromatography adopts a silica gel column, and the eluent is petroleum ether / ethyl acetate=10:1 (v / v)) .
[0042] The method for preparing N-substituted pyrrole derivatives of the present invention has the advantages of wide source of raw materials, cheap and easy to obtain, simple and easy operation, no need for inert gas protection in the whole preparation process, high yield and the like.
Embodiment 1
[0045] Preparation of N-(4-nitrobenzyl)-1H-pyrrole:
[0046]
[0047] Suspend p-nitrobenzaldehyde (0.756g, 5mmol) and 4-hydroxy-L-proline (0.983g, 7.5mmol) in 10mL of redistilled N,N-dimethylformamide (DMF), Stirring and heating to reflux, reacting for about 30min, the reaction solution was cooled naturally, the solvent was evaporated under reduced pressure, and silica gel column chromatography (eluent was petroleum ether / ethyl acetate=10:1 (v / v)) gave N-(4 -Nitro-benzyl)-1H-pyrrole 0.88g, light yellow solid, yield: 87%; mp: 59.9~60.0℃;
[0048] 1 H NMR (400MHz, CDCl 3. )ppm 8.17 (2H, d, J = 8.67Hz), 7.20 (2H, d, J = 8.57Hz), 6.70 (2H, t, J = 1.97, 1.97Hz), 6.25 (2H, t, J = 1.99, 1.99Hz), 5.19(2H, s);
[0049] 13 C NMR (101MHz, CDCl 3 ) ppm 147.46, 145.76, 127.38, 124.02, 121.26, 109.41, 52.56;
[0050] MS, m / z(relative intensity): 202.1(100), 203.1(10.0), 201.1(28.5), 156.1(10.7), 155.1(7.2), 154.1(7.4), 136.0(23.5), 128.1(5.8), 127.1 (4.1), 106.0 (23.2), 90.0 (15....
Embodiment 2
[0052] Preparation of N-(4-nitrobenzyl)-1H-pyrrole:
[0053]
[0054] Suspend p-nitrobenzaldehyde (0.756g, 5mmol) and 4-hydroxyl-L-proline (0.983g, 7.5mmol) in 10mL[bmIm]BF 4 , stirred and heated to 150°C for 30 minutes, the reaction solution was cooled naturally, poured into water after the reaction solution was naturally cooled, extracted with ethyl acetate, dried over anhydrous sodium sulfate, the filtrate was evaporated under reduced pressure to remove the solvent, silica gel column chromatography (elution The solvent is petroleum ether / ethyl acetate=10:1 (v / v)) to obtain 0.81 g of a light yellow solid, and the yield is 80%. mp: 59.9~60.0℃;
[0055] 1 H NMR (400MHz, CDCl 3 )ppm 8.17 (2H, d, J = 8.67Hz), 7.20 (2H, d, J = 8.57Hz), 6.70 (2H, t, J = 1.97, 1.97Hz), 6.25 (2H, t, J = 1.99, 1.99Hz), 5.19(2H, s);
[0056] 13 C NMR (101MHz, CDCl 3. ) ppm 147.46, 145.76, 127.38, 124.02, 121.26, 109.41, 52.56;
[0057] MS, m / z(relative intensity): 202.1(100), 203.1(10.0), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com