High efficiency liquid chromatography method for separating alvimopan and optical isomers thereof
A high-performance liquid chromatography, optical isomer technology, applied in the field of analysis, can solve problems such as high cost and achieve the effect of high separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
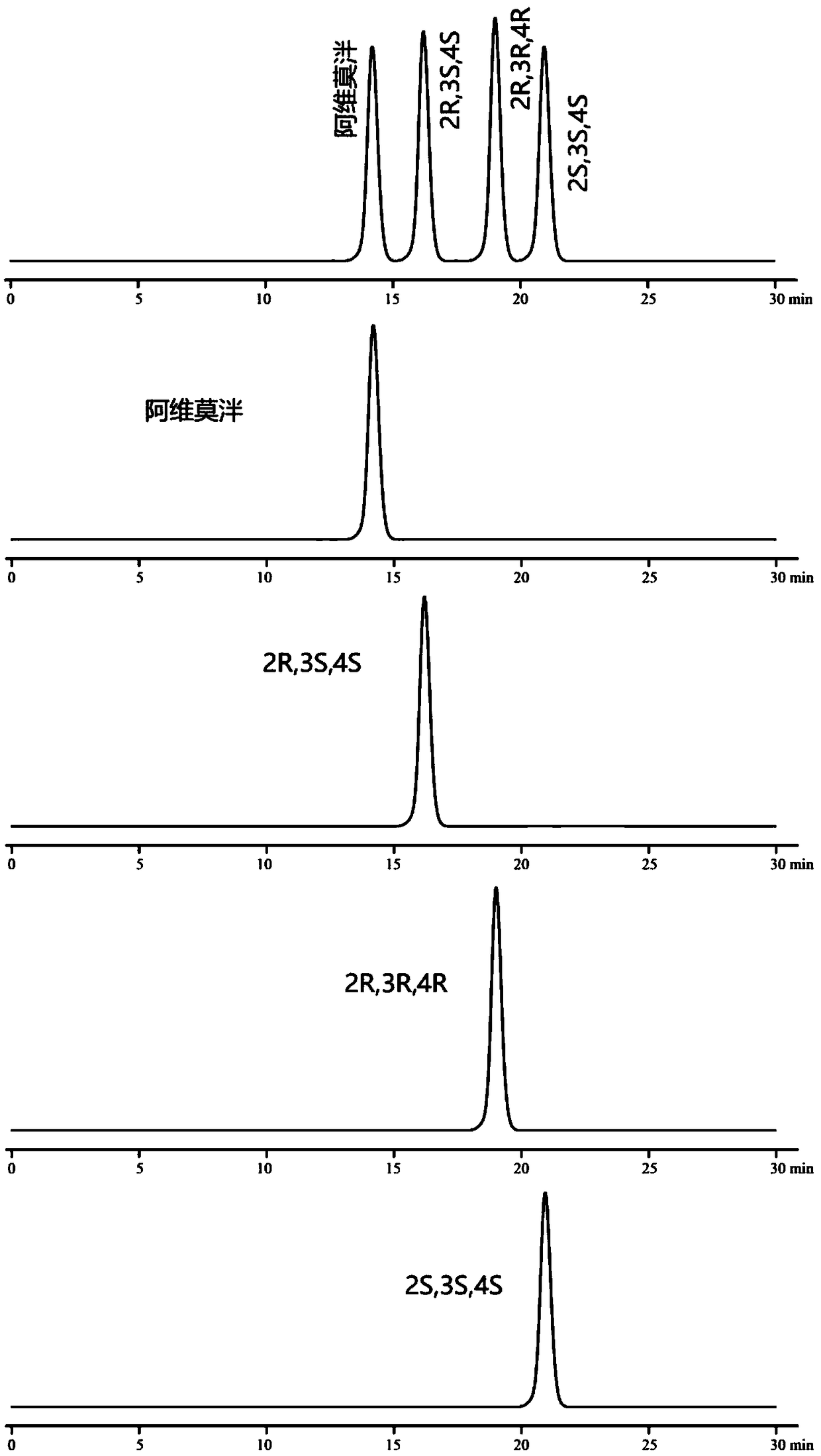
[0070] Embodiment 1: chiral mobile phase method
[0071] 1. Instruments and reagents
[0072] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0073] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0074] 2. Methods and results
[0075] 1. Solution preparation
[0076] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
Embodiment 2
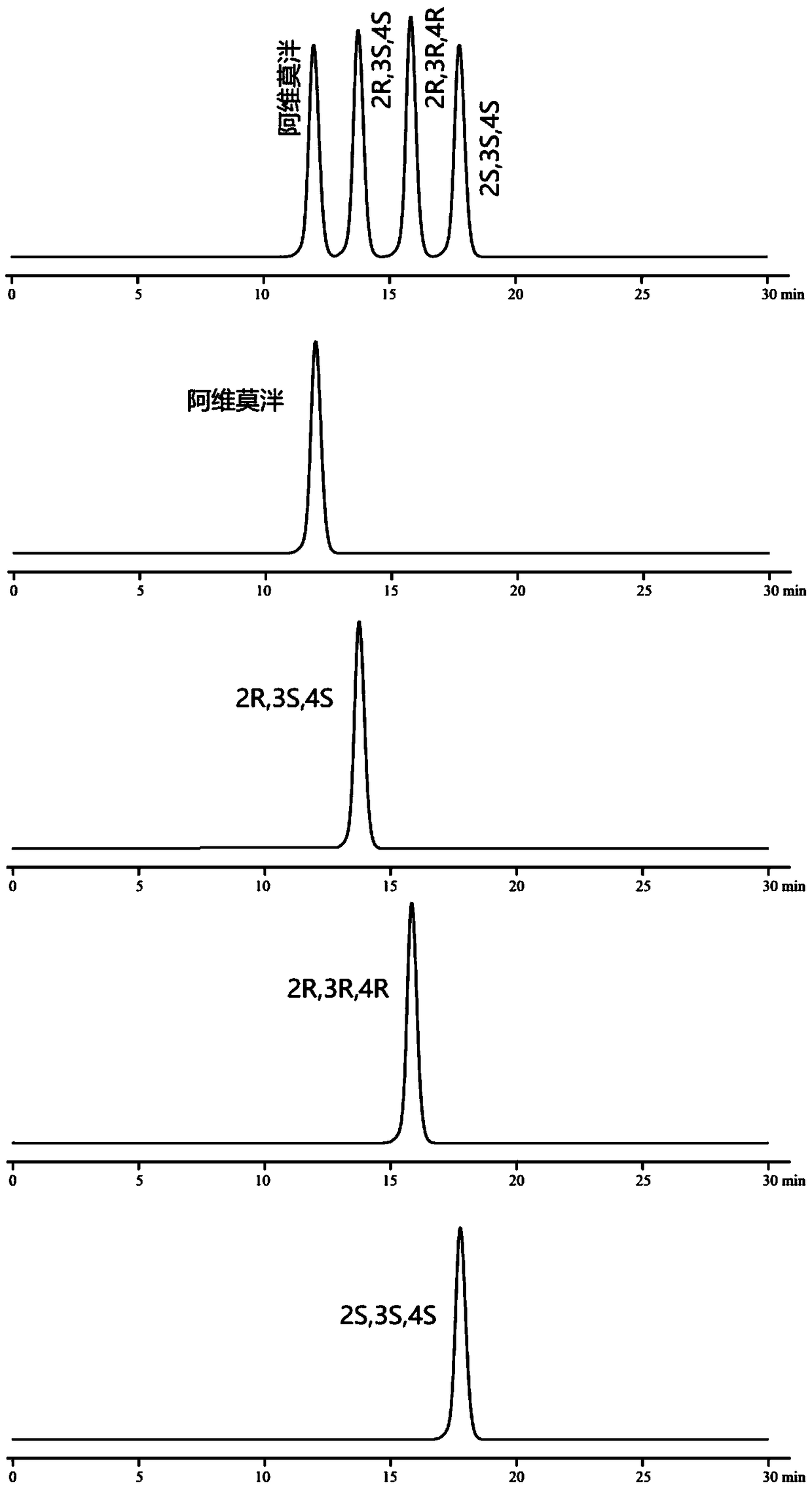
[0088] Embodiment 2: chiral mobile phase method
[0089] 1. Instruments and reagents
[0090] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0091] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0092] 2. Methods and results
[0093] 1. Solution preparation
[0094] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
Embodiment 3
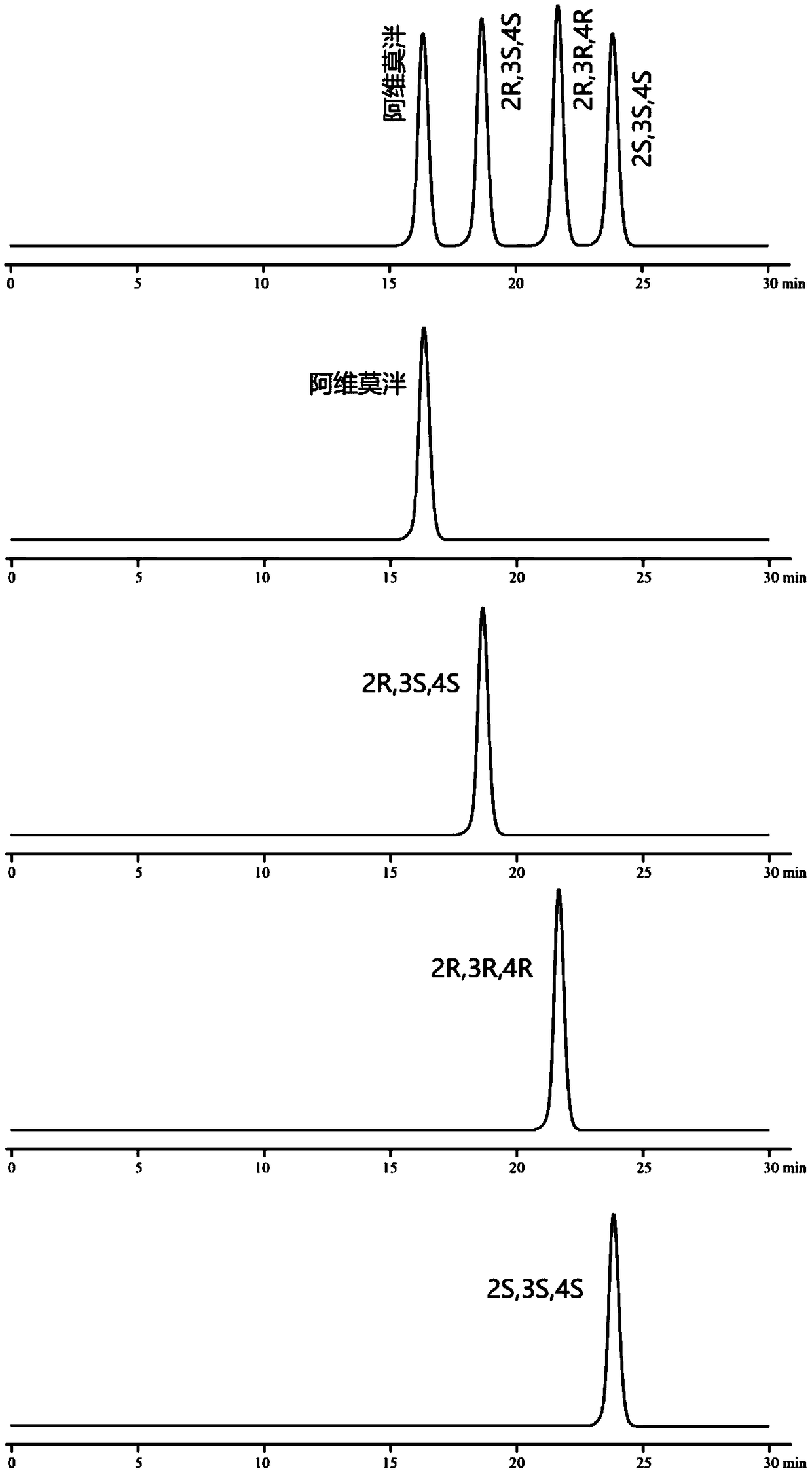
[0106] Embodiment 3: chiral mobile phase method
[0107] 1. Instruments and reagents
[0108] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0109] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0110] 2. Methods and results
[0111] 1. Solution preparation
[0112] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com