HPLC method for separating alvimopan and optical isomers thereof
An optical isomer, dodecanoyl technology, applied in the field of analysis, can solve problems such as high cost and achieve the effect of high separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
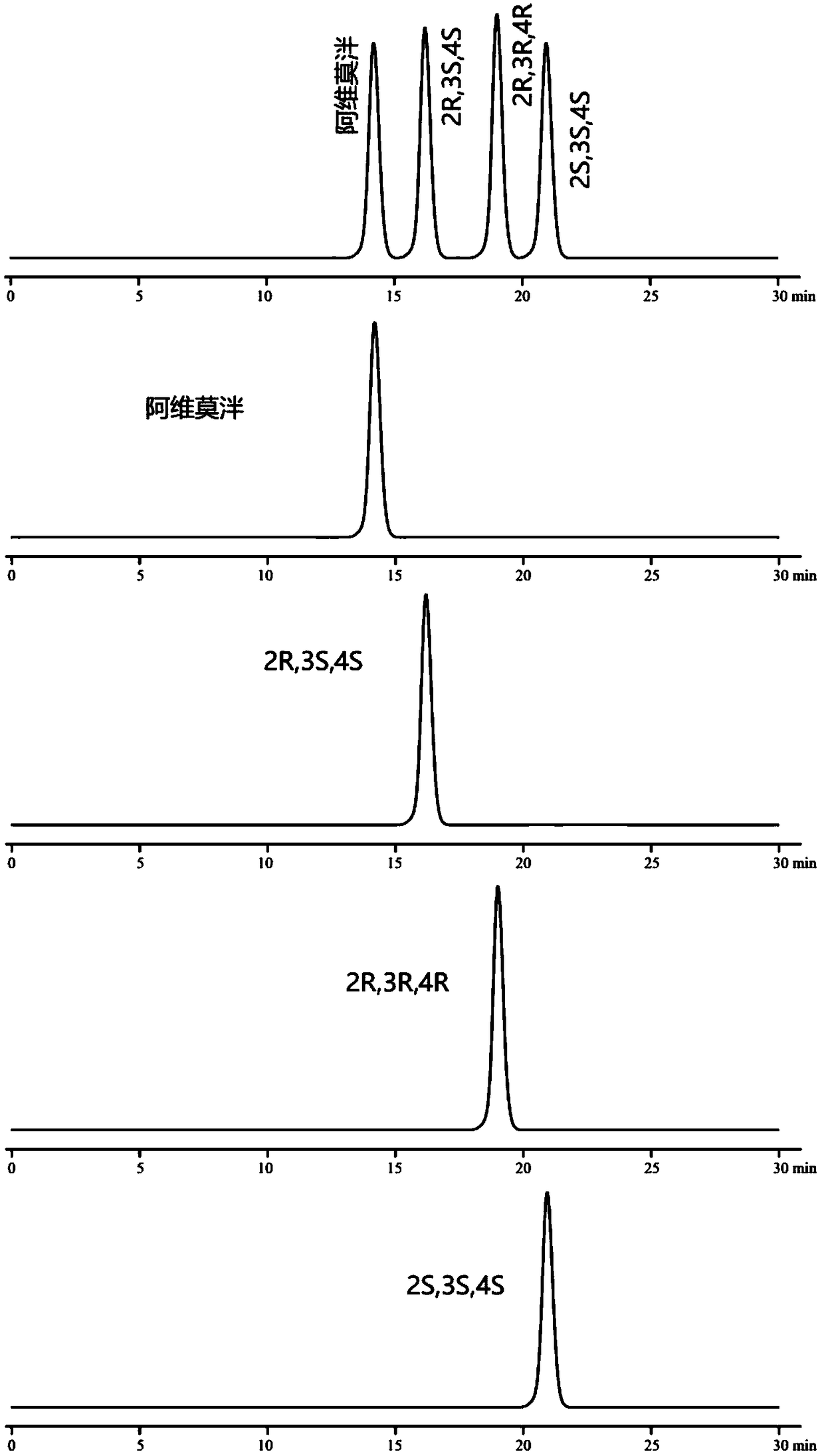
[0070] Embodiment 1: chiral mobile phase method
[0071] 1. Instruments and reagents
[0072] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0073] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0074] 2. Methods and Results
[0075] 1. Solution preparation
[0076] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
Embodiment 2
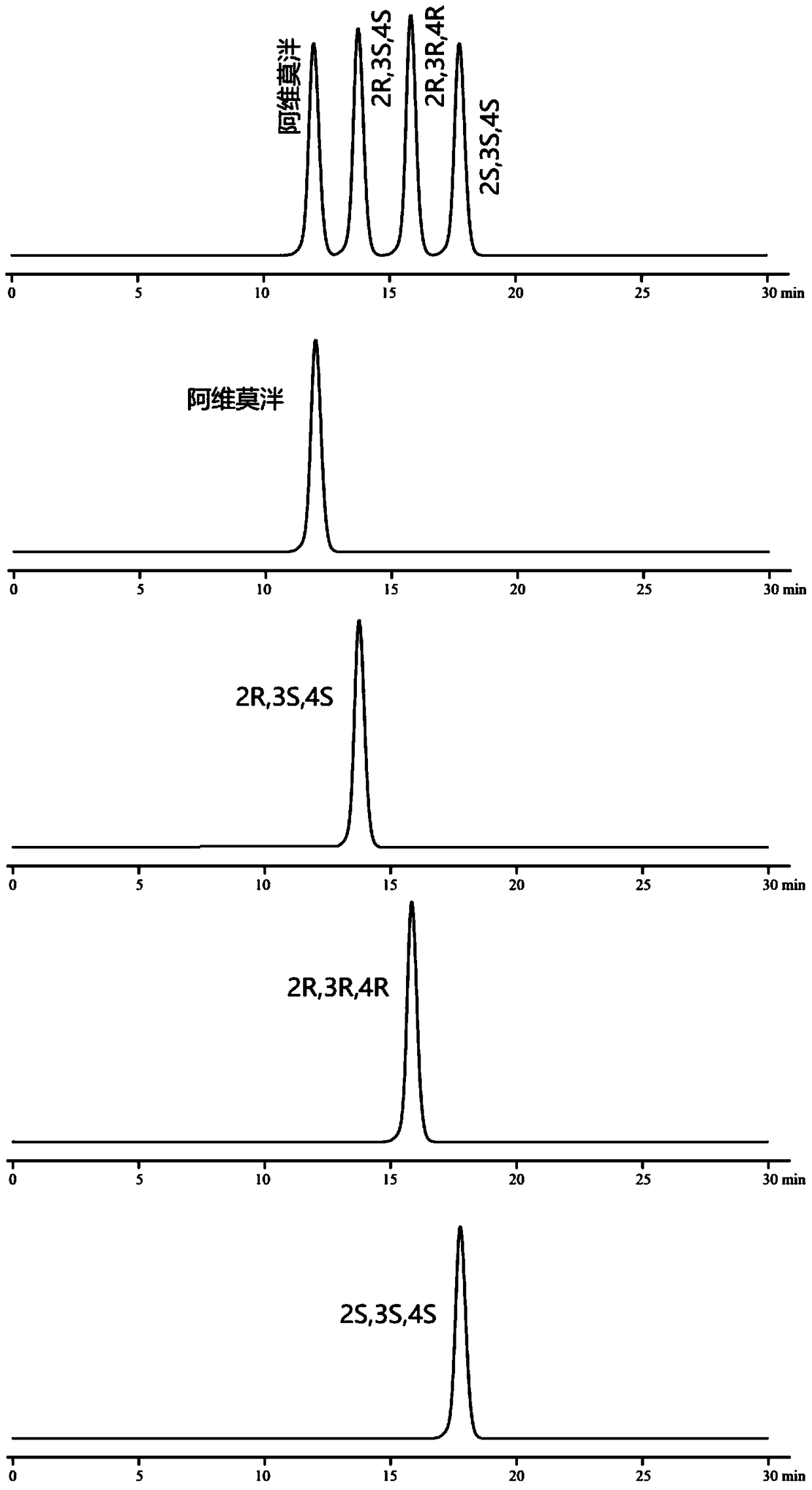
[0088] Embodiment 2: chiral mobile phase method
[0089] 1. Instruments and reagents
[0090] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0091] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0092] 2. Methods and results
[0093] 1. Solution preparation
[0094] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
Embodiment 3
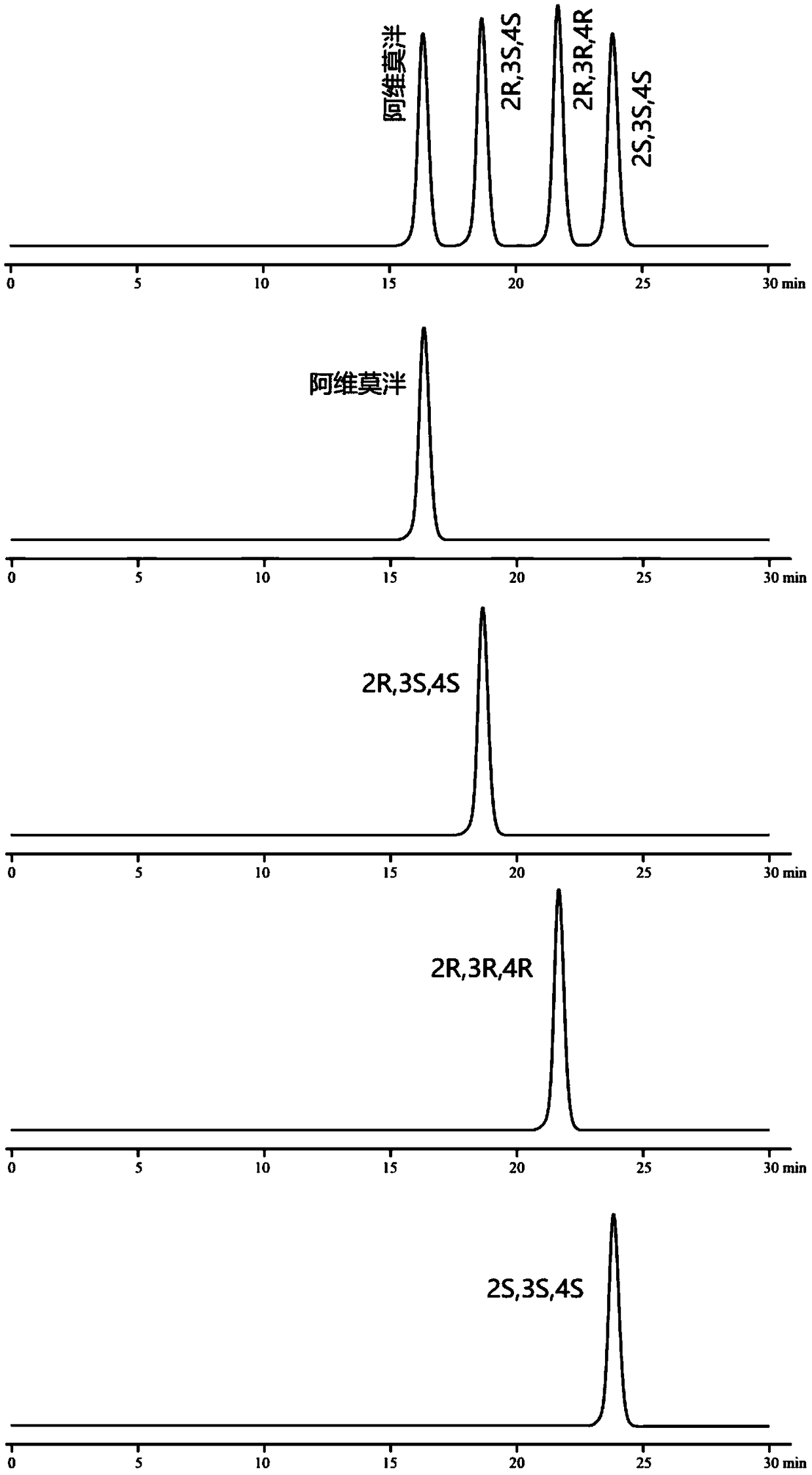
[0106] Embodiment 3: chiral mobile phase method
[0107] 1. Instruments and reagents
[0108] Shimadzu high performance liquid chromatography (Shimadzu LC-20AT pump, SPD-M20A detector, LC solution workstation), XP205 1 / 100,000 electronic balance (METTLER company).
[0109] Acetonitrile and methanol were HPLC grade; water was commercially available Wahaha purified water. Alvimopan reference substance (mass fraction 98.7%), Alvimopan enantiomer reference substance (mass fraction 97.8%), Alvimopan diastereomer (2S, 3S, 4S) reference substance (mass fraction 99.7%), alvimopan diastereoisomers (2R, 3R, 4R) reference substances (mass fraction 89.6%) were purchased from Tianjin Taipu Pharmaceutical Technology Development Co., Ltd.
[0110] 2. Methods and Results
[0111] 1. Solution preparation
[0112] Monomer reference solution: Accurately weigh the appropriate amount of alvimopan, enantiomers, diastereoisomers (2R, 3R, 4R), (2S, 3S, 4S), and prepare them respectively with mobi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com