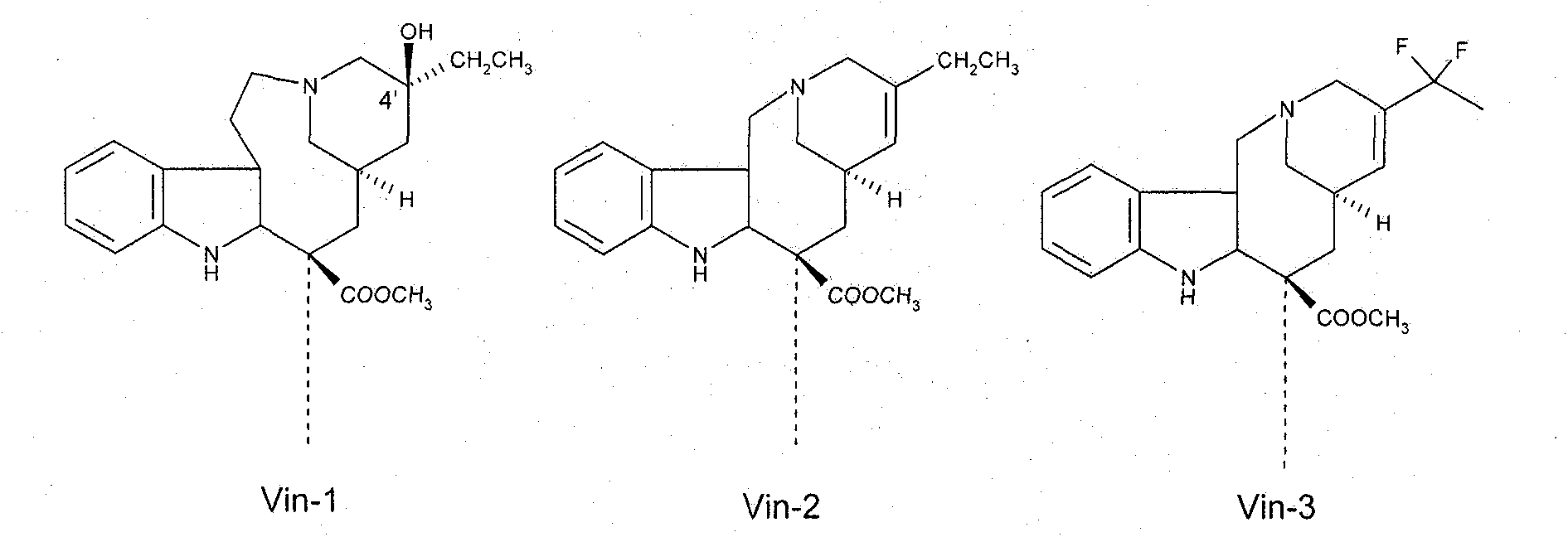
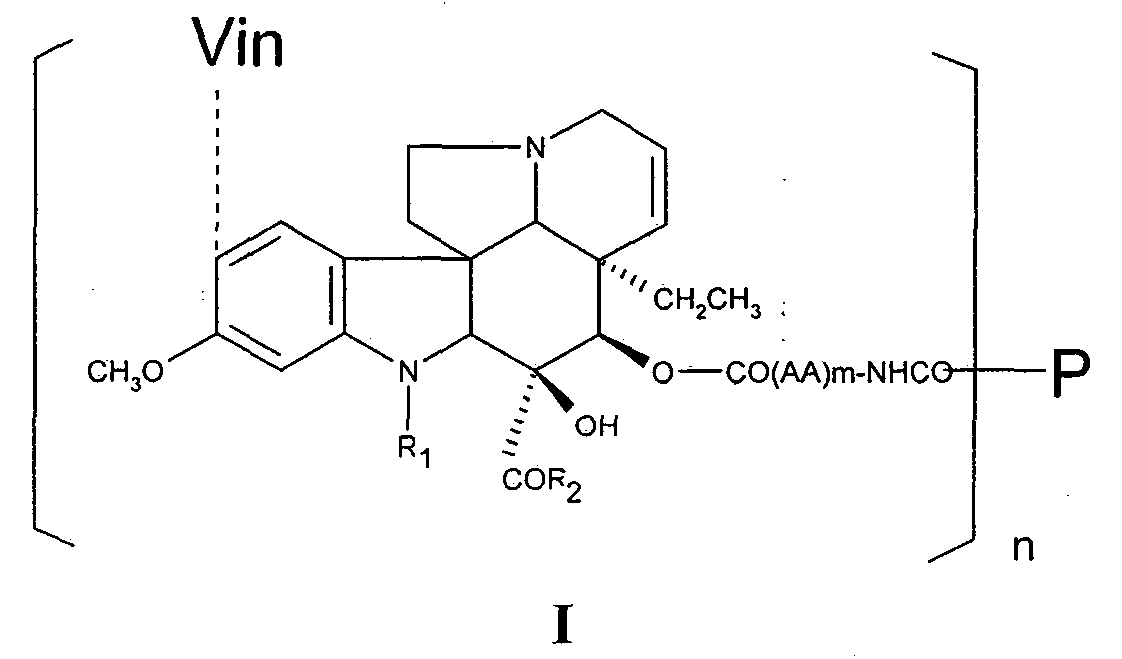
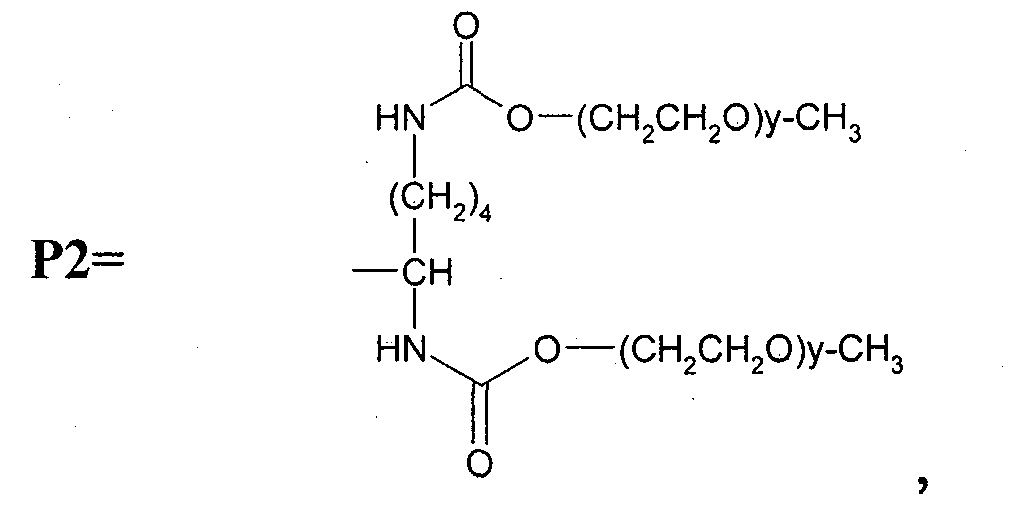
Macromolecule vinblastine conjugate adopting amino acids or oligopeptides as connexons
A conjugate and macromolecular technology, applied in the field of macromolecules-vinblastine conjugates with amino acids or oligopeptides as linkers, can solve the problem of large adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1 monomethoxypolyethylene glycol 10kD -[4-O-(leucyl)-desacetylvinblastine]-conjugate (m-PEG 10kD -OCH 2 CH 2 CONH-Leu-CO-des-vinblastine, I 1 ) preparation
[0110] 1.1 Synthesis of 4-deacetylvinblastine (des-vinblastine)
[0111] Put 875ml of anhydrous methanol in a 3000ml three-necked flask, put it in an ice-salt bath, and pass through dry hydrogen chloride gas for 4 hours continuously to prepare a saturated hydrogen chloride methanol solution (0°C). Add 8g of vinblastine sulfate, continue to feed hydrogen chloride gas, react at low temperature, the reaction solution first becomes turbid and then clarifies, TLC detects the degree of progress of the reaction (developing agent DCM:CH 3 OH=15:1,R f =0.45), the reaction was carried out for 72h, and the reaction was stopped. Concentrate under reduced pressure to remove the solvent, leaving a viscous solid, dissolve it in water, add crushed ice, and then adjust the pH with ammonia water until no more preci...
Embodiment 2
[0117] Embodiment 2 monomethoxypolyethylene glycol 10kD -[4-O-(leucyl)-desacetylvincristine] conjugate (m-PEG 10kD -OCH 2 CH 2 CONH-Leu-CO-des-vincristine, I 2 ) preparation
[0118] 2.1 Synthesis of 4-deacetyl vincristine (des-vincristine)
[0119] Put 875ml of anhydrous methanol in a 3000ml three-necked flask, put it in an ice-salt bath, and pass through dry hydrogen chloride gas for 4 hours continuously to prepare a saturated hydrogen chloride methanol solution (0°C). Add 8g of vincristine, continue to feed hydrogen chloride gas, react at low temperature, the reaction solution first becomes turbid and then clarifies, TLC detects the degree of progress of the reaction (developing agent DCM:CH 3 OH=15:1, R f =0.45), the reaction was carried out for 72h, and the reaction was stopped. Concentrate under reduced pressure to remove the solvent, leaving a viscous solid, dissolve it in water, add crushed ice, and then adjust the pH with ammonia water until no more precipitates ...
Embodiment 3
[0125] Embodiment 3 monomethoxypolyethylene glycol 10kD -[4-O-(leucyl)-deacetylvinorelbine] conjugate (m-PEG 10kD -OCH 2 CH 2 CONH-Leu-CO-des-vinorelbine, I 3 ) preparation
[0126] 3.1 Synthesis of 4-deacetyl Vinorelbine (des-vinorelbine)
[0127] Put 875ml of anhydrous methanol in a 3000ml three-necked flask, put it in an ice-salt bath, and pass through dry hydrogen chloride gas for 4 hours continuously to prepare a saturated hydrogen chloride methanol solution (0°C). Add 8g Vinorelbine, continue to pass into hydrogen chloride gas, react at low temperature, the reaction solution first becomes turbid and then clarifies, TLC detects the degree of progress of the reaction (developing agent DCM:CH 3 OH=15:1,R f =0.45), the reaction was carried out for 72h, and the reaction was stopped. Concentrate under reduced pressure to remove the solvent, leaving a viscous solid, dissolve it in water, add crushed ice, and then adjust the pH with ammonia water until no more precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com