Cerium and/or terbium phosphate, optionally with lanthanum, phosphor resulting from said phosphate, and methods for making same
A light-emitting material, phosphate technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0071] For the first embodiment, a minimum lithium content is not necessary and it may correspond to the lowest value detectable by the analytical technique used to measure this content. However, this minimum lithium content is generally at least 10 ppm, more particularly at least 90 ppm.
[0072] Phosphates of the monazite crystal structure consist of microparticles which themselves consist of aggregates of crystallites with a size measured in the plane (012) of at least 70 nm, more particularly of at least 80 nm, which size may also vary with the precursor in It varies with the calcination temperature experienced during its preparation. Here again, as before, it can be observed that the phosphates according to the invention have better crystallinity than the phosphates of the prior art with the same structure.
[0073] For those phosphates that have been subjected to calcination or heat treatment at temperatures generally above 600°C, advantageously 800 to 900°C, although t...
Embodiment 2
[0154] This example relates to the preparation of lanthanum, cerium and terbium phosphates according to the invention.
[0155] Within 1 h, to 1 L containing 1.5 mol / L analytical grade phosphoric acid H previously adjusted to pH 1.6 by addition of LiOH and adjusted to 60 °C 3 PO 4 Add 1 liter of rare earth metal chloride solution of 4N purity to the solution, the solution of rare earth metal chloride has an overall concentration of 1.3 mol / liter, and can be subdivided as follows: 0.57 mol / liter of lanthanum chloride, 0.56 mol / liter of Cerium chloride and terbium chloride at 0.17 mol / l. The pH was adjusted to 1.6 by adding LiOH during the precipitation.
[0156] At the end of the precipitation step, the mixture was maintained at 60°C for an additional 15 minutes. The resulting precipitate was then recovered by filtration, washed with water, then dried in air at 60°C, and then subjected to heat treatment in air at 840°C for 2 hours. At the end of the calcination, the product...
Embodiment 4
[0165] This example relates to the preparation according to the invention of a phosphor obtained from the phosphate of Example 2.
[0166] The precursor phosphate obtained in Example 2 was reprocessed under the same conditions as in Example 3.
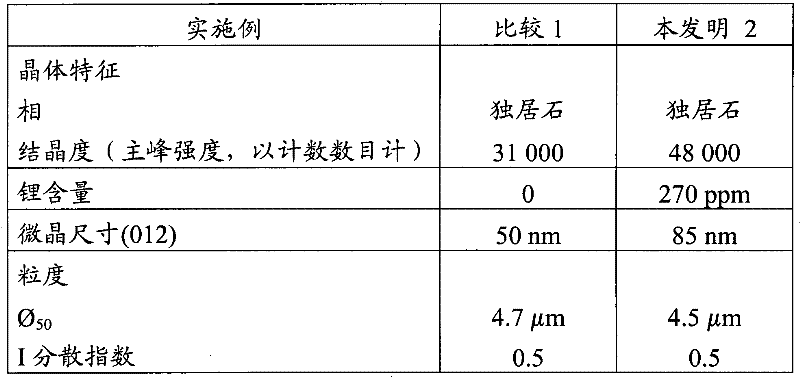
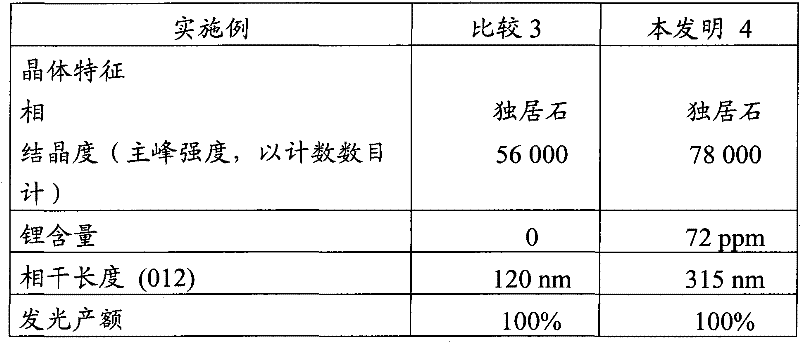
[0167] The characteristics of the products of Examples 3 and 4 are listed in Table 2 below.
[0168] Table 2
[0169]
[0170] The luminescence yield of product 4 according to the invention is given relative to comparative product 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com