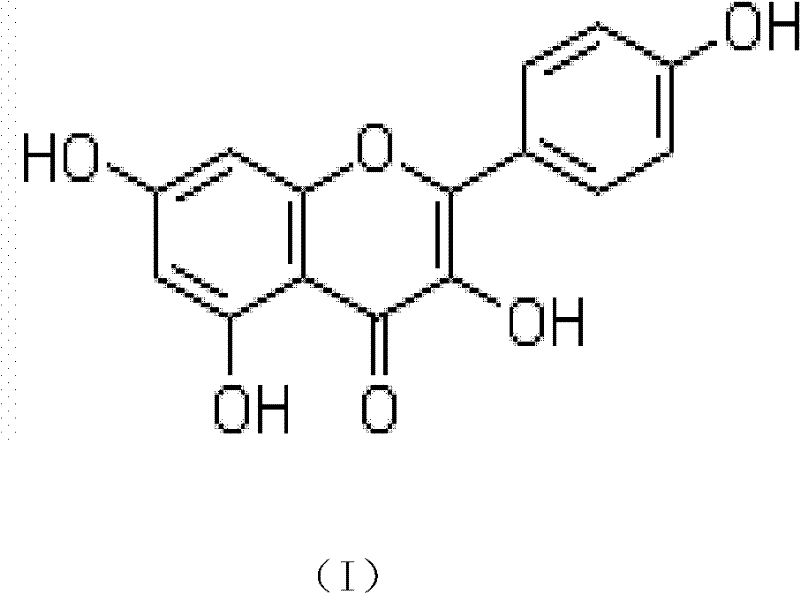
Application of kaempferol as synergist of anti-fungal medicaments
A technology of antifungal drugs and synergists, applied in the field of medicine, to achieve the effects of reducing toxic side effects and treating fungal infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
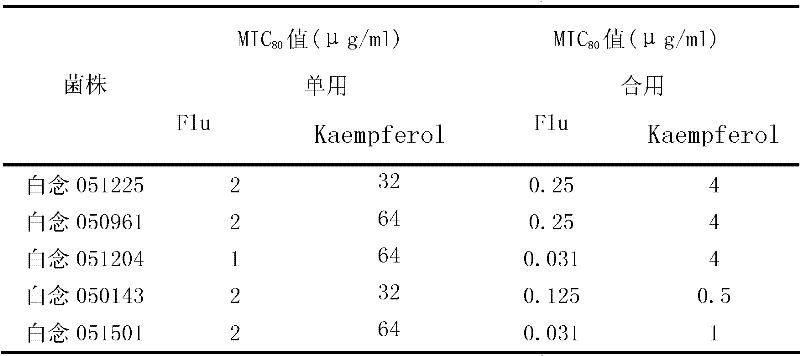
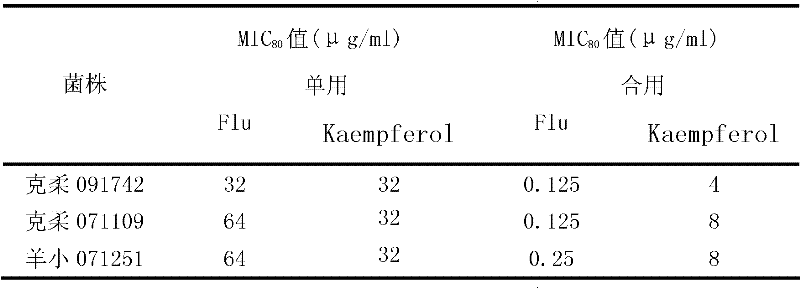
[0010] Example 1: Effects of combined use of kaempferol and fluconazole on different clinical fungal strains.
[0011] Materials and methods
[0012] 1. Test drug:
[0013] Kaempferol: China Institute for the Control of Pharmaceutical and Biological Products (the same below).
[0014] Fluconazole: Pfizer Pharmaceutical Co., Ltd. (the same below).
[0015] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0016] The concentration of kaempferol was 8 mg / ml, the concentration of fluconazole was 2 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0017] 2. Strains:
[0018] The clinical strains of Candida albicans, Candida krusei and Microsporum lanoides were provided by the fungal laboratory of Shanghai Changhai Hospital, and were identified by morphology and biochemi...
Embodiment 2
[0046] Embodiment 2: the effect of kaempferol combined with fluconazole on different clinical and laboratory induced drug-resistant strains
[0047] Materials and methods
[0048] 1. Test drug:
[0049] The sources of kaempferol and fluconazole are the same as above.
[0050] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0051] The concentration of kaempferol was 8 mg / ml, the concentration of fluconazole was 2 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0052] 2. Strains:
[0053] Clinical drug-resistant strain: Candida albicans, provided by the Fungi Department of Shanghai Changhai Hospital, confirmed by morphological and biochemical identification.
[0054] Drug-resistant strains induced in the laboratory: Candida albicans (SC5314-R, Y01-R) are drug-resis...
Embodiment 3
[0062] Embodiment 3: the combination medicine of kaempferol and miconazole, ketoconazole
[0063] Materials and methods
[0064] 1. Test drug:
[0065] Kaempferol: National Institute for the Control of Pharmaceutical and Biological Products.
[0066] Fluconazole: Pfizer Pharmaceuticals Ltd.
[0067] Ketoconazole: National Institute for the Control of Pharmaceutical and Biological Products.
[0068] Miconazole: National Institute for the Control of Pharmaceutical and Biological Products.
[0069] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0070] The concentration of kaempferol was 8 mg / ml, the concentration of miconazole and ketoconazole was 6.4 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0071] Other experimental steps and methods are the same as in E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com