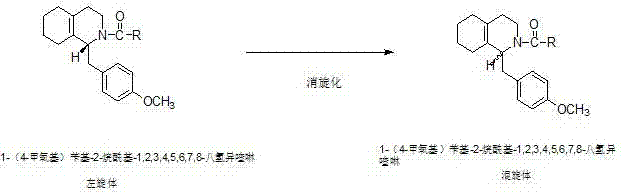
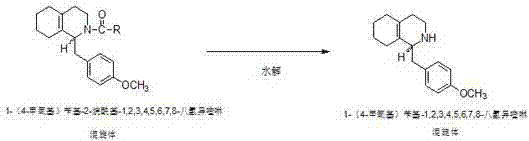
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
A technology of octahydroisoquinoline and hydrobromic acid, which is applied in organic chemistry and other fields, and can solve the problems of low utilization rate and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer):
[0023] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times molar amount of acetic anhydride, nitrogen protection, heating up to 80°C, heat preservation reaction for 3 hours, after heat preservation reaction is completed, vacuum distillation, after distillation is complete, add 2 mole amount of absolute ethanol Recrystallized, centrifuged, and dried to obtain light yellow solid 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline ( L-isomer) 142.8 g, the yield was 81.8%.
[0024] Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0025] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 142.8g of 1-(4-methoxy)be...
Embodiment 2
[0029] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer).
[0030] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times molar amount of acetic acid, nitrogen protection, heating to 80 ° C, heat preservation reaction for 4 hours, heat preservation reaction is completed, vacuum distillation, after distillation, add 2 mole weight of absolute ethanol Crystallized, centrifuged, and dried to obtain light yellow solid 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (Levorotatory body) 143.4 g, the yield was 82.1%.
[0031] Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0032] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, and add 143.4 g of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5...
Embodiment 3
[0036] Step 1: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (L-isomer):
[0037] In a 500ml four-neck flask equipped with a thermometer and a stirring device, start stirring, and add 150g of 1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-octahydro Isoquinoline (L-isomer), 1.1 times the molar amount of formic acid, pass through nitrogen protection, heat up to 80 ° C, heat preservation reaction for 3 hours, heat preservation reaction is completed, vacuum distillation, after distillation is completed, add 2 moles of anhydrous methanol Crystallized, centrifuged, and dried to give light yellow solid 1-(4-methoxy)benzyl-2-formyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (Levorotatory body) 134.4 g, the yield was 80.8%.
[0038] Step 2: Preparation of 1-(4-methoxy)benzyl-2-acetyl-1,2,3,4,5,6,7,8-octahydroisoquinoline (heteromorph):
[0039] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, and add 134.4 g of 1-(4-methoxy)ben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com