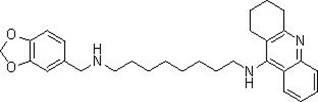
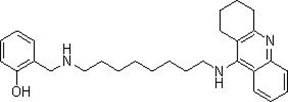
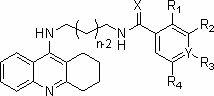
Tacrine heterocomplex, preparation method and use in medicines for curing neurodegenerative diseases thereof
A technology of tacrine and hybrids, applied in the field of medicine and chemical industry, to achieve the effects of high inhibition, high changes in the aggregation properties of β-amyloid protein, and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] N-(1,2,3,4-tetrahydroacridin-9-amino)octyl-isonicotinamide.
[0043] N 1 -(1,2,3,4-tetrahydroacridine-9-amino)butyl-1,8-octanediamine (1.0 mmol) was dissolved in anhydrous dichloromethane, and pyridine-4-carboxylic acid was added under stirring (1.0 mmol), N , N '-Isopropylcarbodiimide (DIC, 1.1 mmol) and 4-dimethylaminopyridine (DMAP, 0.5 mmol), stirred overnight at room temperature. Wash with water, take the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and separate the residue by column chromatography. The eluent is chloroform:methanol:ammonia=40:1:0.5%. A white solid was obtained.
[0044] Yield: 50%, m.p. 90-92℃, 1 H NMR (400 MHz, CDCl 3 ) δ 8.62 (d, J = 6.0 Hz, 2H), 7.96 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 8.4 Hz, 1H), 7.51 (t, J = 7.6 Hz, 1H), 7.32 (s, 1H), 3.85 (s, 1H), 3.47 (t, J = 7.2 Hz, 2H), 3.38 (dd, J = 13.8, 6.6 Hz, 2H), 3.00 (s, 2H), 2.67 (s, 2H), 1.87 (t, J = 3.0 Hz, 4H),...
Embodiment 2
[0046]
[0047] N 1 -(pyridine-4-methylene)-N 8 -(1,2,3,4-Tetrahydroacridin-9-amino)octyl-1,8-octanediamine.
[0048] N 1 -(1, 2, 3, 4-tetrahydroacridine-9-amino)butyl-1,8-octanediamine (1.0mmol) was dissolved in anhydrous methanol, and pyridine-4-carboxaldehyde (1.0 mmol), after stirring overnight at room temperature, sodium borohydride (6.0 mmol) was added under cooling in an ice bath, and the reaction was allowed to rise to room temperature naturally for 8 hours. After concentration, add water, extract with ethyl acetate, dry, concentrate, and separate by column chromatography. The eluent uses chloroform:methanol:ammonia=35:1:0.5%. A pale yellow oily liquid was obtained.
[0049] Yield: 52%, 1 H NMR (400 MHz, CDCl 3 ) δ 8.50 (d, J = 6.0 Hz, 2H), 7.52 (t, J = 7.6 Hz, 1H), 7.34 – 7.29 (m, 1H), 7.23 (d, J = 5.9 Hz, 2H), 3.76 (s, 2H), 3.47 (t, J = 7.2 Hz, 2H), 3.05 (s, 2H), 2.67 (s, 2H), 2.57 (t, J = 7.2 Hz, 2H), 1.88 (t, J = 3.2 Hz, 4H), 1.71 – 1.57 (m,...
Embodiment 3
[0051]
[0052] 4-{6-(1,2,3,4-tetrahydroacridin-9-amino)hexylamino-methylene}phenol.
[0053] Method is the same as embodiment two, and difference is to use N 1 -(1,2,3,4-tetrahydroacridin-9-amino)hexyl-1,6-hexanediamine instead of N 1 -(1, 2, 3, 4-tetrahydroacridine-9-amino)octyl-1,8-octanediamine, p-hydroxybenzaldehyde instead of pyridine-4-carbaldehyde, and finally a light yellow oily liquid was obtained.
[0054] Yield: 33%, 1 H NMR (400 MHz, CDCl 3 ) δ 7.93 (t, J = 9.1 Hz, 2H), 7.49 (t, J = 7.3 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.07 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.4 Hz, 2H), 5.98 (s, 1H), 4.09 (s, 1H), 3.66 (s, 2H), 3.47 (t, J = 7.2 Hz, 2H), 3.04 (s, 2H), 2.72 – 2.51 (m, 4H), 1.86 (d, J = 2.8 Hz, 4H), 1.69 – 1.56 (m, 2H), 1.57 – 1.45 (m, 2H), 1.32 (dd, J = 6.3, 3.2 Hz, 4H). 13 C NMR (100 MHz, CDCl 3) δ 157.98, 157.20, 151.33, 146.64, 129.66, 129.26, 128.70, 127.66, 123.70, 123.01, 119.84, 115.89, 115.30, 53.21, 49.28, 48.72, 33.11, 31.62,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com