Potassium citrate sustained release tablet and preparation method thereof
A technology of potassium citrate and sustained-release tablets, which is applied in the fields of pharmaceutical formulation and drug delivery, which can solve the problems of hidden dangers in product quality control and large measurement errors, and achieve the effects of stable and controllable quality, improved release rate, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
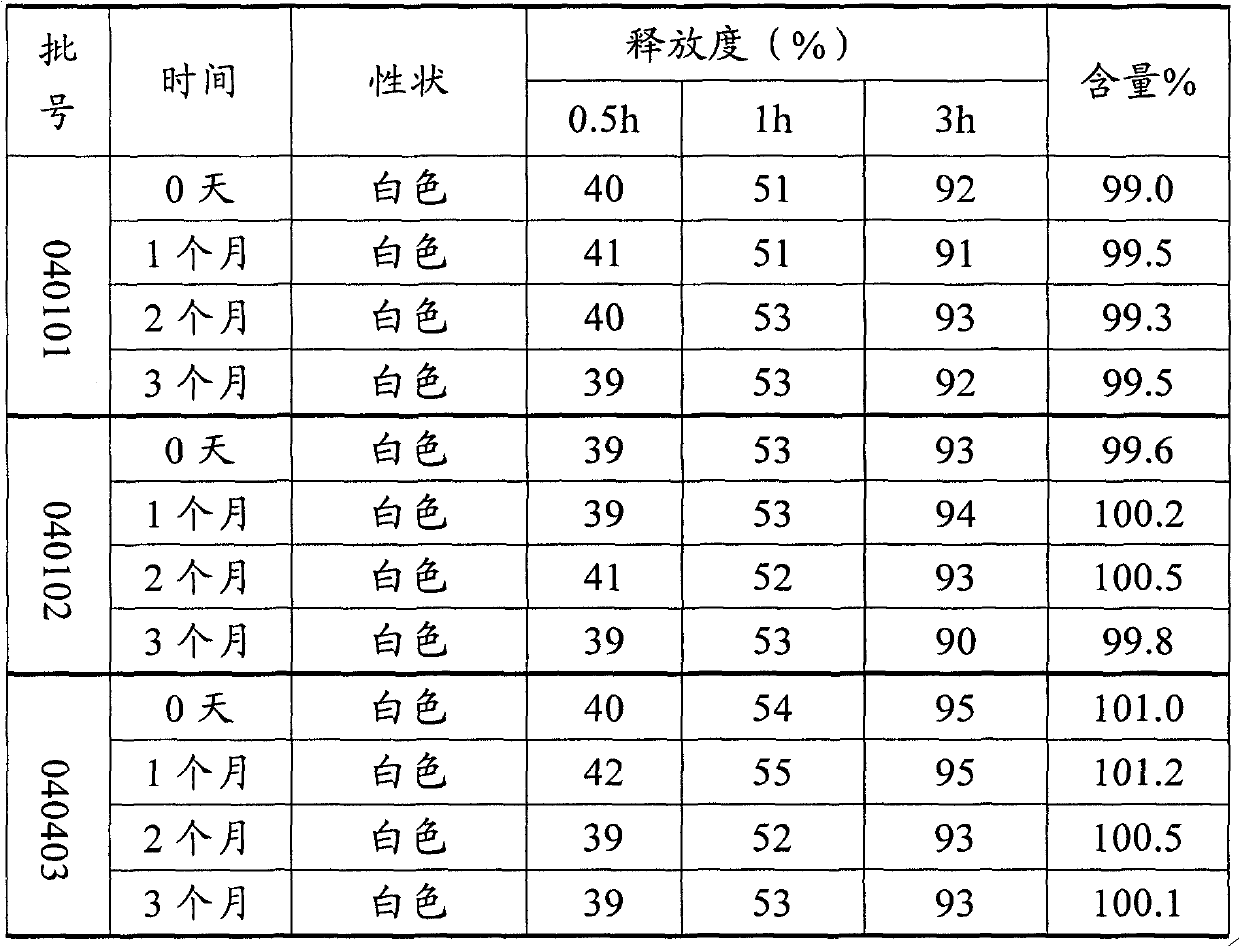
[0051] Prescription optimization: On the basis of the Urocit-K prescription and its stability test results, screen the dosage of the sustained-release matrix in the prescription, so as to control the release of the drug in the sustained-release tablets more effectively and improve the stability of the sustained-release tablets. 3 hours release.
[0052] The preparation process of sustained-release tablets is as follows:
[0053] (1) Mix and pulverize the potassium citrate crude drug and 2 / 3 of the prescription amount of micropowder silica gel, pass through an 80-mesh sieve, and set aside;
[0054] (2) Weighing the mixture of above-mentioned potassium citrate and micropowder silica gel, and glyceryl behenate were placed in a blender and mixed for 10 minutes;
[0055] (3) Add the mixture in step (2) into the jacketed heating pot, adjust the temperature until the material temperature is 80-90°C, stir slowly, and melt for 30 minutes;
[0056] (4) After being cooled to room tempe...
Embodiment 2
[0066] Further optimization of the formulation: In order to further adjust the release rate, it is planned to add crospovidone during the melt granulation step on the basis of formulation 2.
[0067] According to the preparation process described in Example 1, the sustained-release tablet samples were prepared and the release rate was tested. The results are shown in Table 2.
[0068] Table 2 Composition and release results of each formulation
[0069]
[0070] According to the prescription screening results, adding a certain amount of crospovidone further improved the 3-hour release of the sustained-release tablet, wherein when the mass ratio of glyceryl behenate to crospovidone was 2:1 (that is, the prescription 2c), the release results are the best.
Embodiment 3
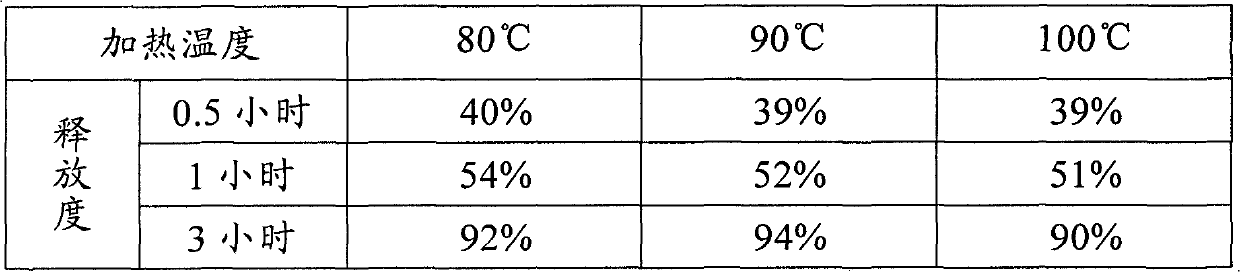
[0072] Screening of preparation process parameters: the slow-release matrix glyceryl behenate has a melting point of 75-80°C, and the melting temperature recommended by the excipient manufacturer is 80-85°C. Considering that the different heating temperatures of melt granulation may lead to differences in the molecular arrangement of the sustained-release material, thereby affecting the release rate, three heating temperatures were initially set: 80°C, 90°C, and 100°C, according to prescription 2c and Example 1. The preparation method prepares the sustained-release tablet sample and detects the release rate, and the results are shown in Table 3.
[0073] Table 3 Melt granulation heating temperature and release results
[0074]
[0075] From the above results, it can be seen that there is no significant difference in the release degree of the three samples. Considering the energy efficiency and equipment parameter control error, it is preferable to set the heating temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com