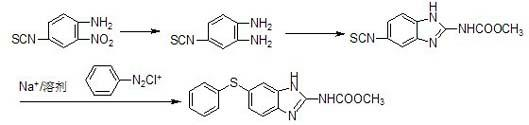
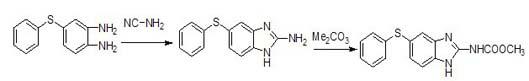
Preparation method for anthelmintic benzimidazole fenbendazole
A technology of benzimidazole and benzimidazole, which is applied in the field of preparation of anti-parasitic raw material drug benzimidazole, can solve the problems of difficult operation, great impact on the environment, and high cost, and achieve the effect of safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
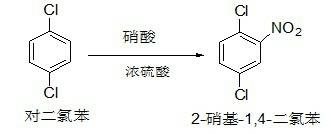
[0032] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0033] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, add 120.0 g of p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, and drop nitric acid with a molar weight of 1.04 times that of dichlorobenzene, and control the dropping temperature At 20-25°C, the dropwise addition is completed, and the reaction is kept at 35-40°C for 1 hour, and the GC tracks the reaction completely, and the lower waste acid layer is separated; the weight ratio of p-dichlorobenzene is added to purify and wash, and the water layer is separated; Neutralize to neutral with liquid caustic soda, discard the water layer. 153.1 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. This step yield is 97.7%.
[0034] Step 2: Preparation of 2-nitro-4-chloroaniline
[0035] 153.1g of 2-nitro-1,4-dichlorobenzene obtained in the previous step, 2.2 ti...
Embodiment 2
[0043] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0044] In a 500 ml four-necked flask with a thermometer and a stirring device, start stirring, add 120.0 g of p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, and drop nitric acid with a molar weight of 1.04 times that of dichlorobenzene, and control the dropping temperature At 20-25°C, the dropwise addition is completed, and the reaction is kept at 35-40°C for 1 hour, and the GC tracks the reaction completely, and the lower waste acid layer is separated; the weight ratio of p-dichlorobenzene is added to purify and wash, and the water layer is separated; Neutralize to neutral with liquid caustic soda, discard the water layer. 153.4 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. This step yield is 97.9%.
[0045] Step 2: Preparation of 2-nitro-4-chloroaniline
[0046]153.4g of 2-nitro-1,4-dichlorobenzene obtained in the previous step, 2.2 tim...
Embodiment 3
[0054] Step 1: Preparation of 2-nitro-1,4-dichlorobenzene
[0055] In a 1000 ml four-necked flask with a thermometer and a stirring device, start stirring, add 240.0 g of p-dichlorobenzene, 0.8 times the weight ratio of concentrated sulfuric acid, dropwise add nitric acid with a molar mass of 1.04 times that of p-dichlorobenzene, and control the dropwise Adding temperature is 20-25°C, dropwise is completed, keep warm at 35-40°C for 1 hour, GC tracks the reaction is complete, remove the lower waste acid layer; add p-dichlorobenzene at a weight ratio of 1 times for purification and washing, and remove the water layer ; Then use liquid caustic soda to neutralize, discard the water layer. 307.2 g of 2-nitro-1,4-dichlorobenzene was obtained, which was directly used in the next reaction. This step yield is 98.0%.
[0056] Step 2: Preparation of 2-nitro-4-chloroaniline
[0057] 307.2g of 2-nitro-1,4-dichlorobenzene obtained in the previous step, 2.2 times the weight ratio of 2-ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com