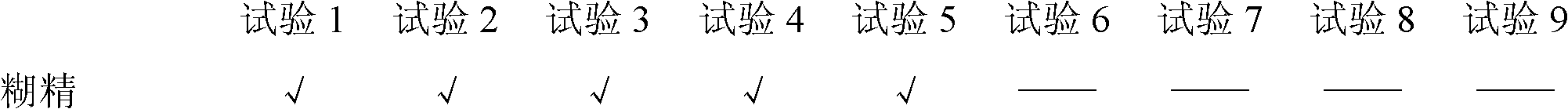Cefadroxil chewable tablets and preparation method thereof
A cefadroxil chewable tablet, cefadroxil technology, applied in the direction of medical formula, non-active ingredient medical preparations, medical preparations containing active ingredients, etc., can solve the problem of not giving, discomfort, lack of excipients and Issues such as the technical inspiration of its dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
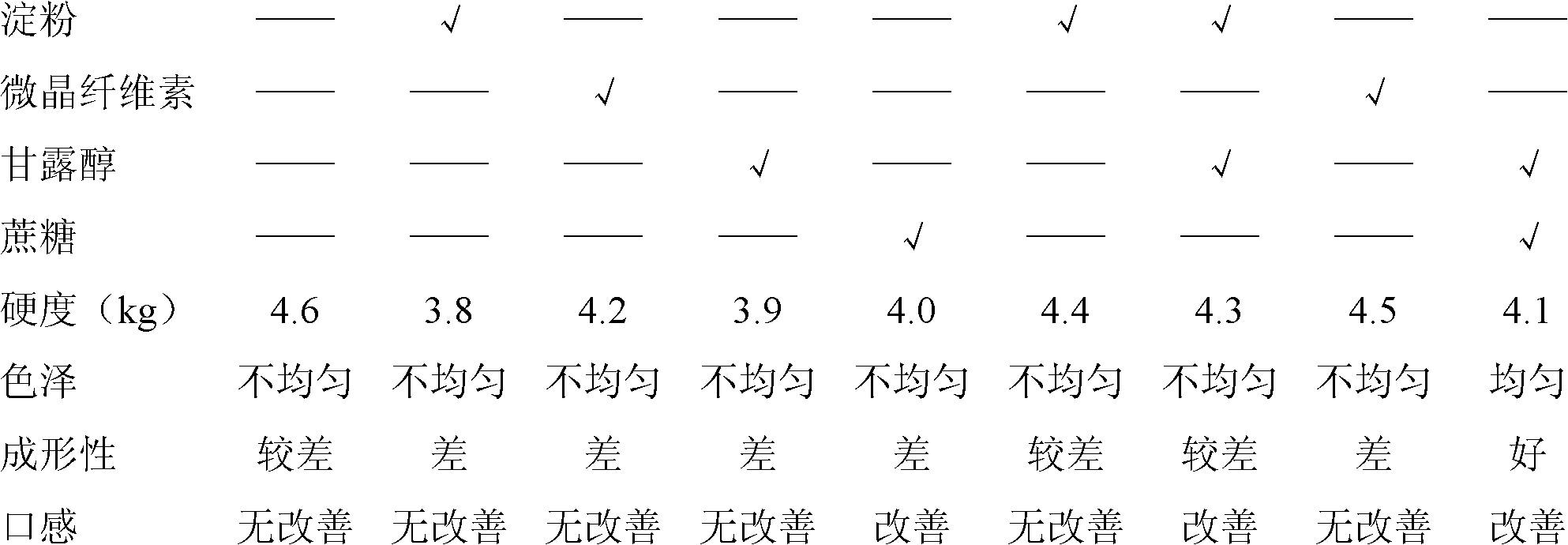
Image
Examples
Embodiment 1
[0092] (1) Prescription
[0093] Ingredients 1000 pieces (g)
[0094] Cefadroxil 132g (equivalent to cefadroxil anhydrous 125g)
[0095] Mannitol 250g
[0096] Sucrose 123g
[0097] Maltodextrin 22g
[0098] Aspartame 1g
[0099] Sweet Orange Powder Flavor 15g
[0100] Menthol 1g
[0101] Appropriate amount of water
[0102] Magnesium Stearate 5.5g
[0103] (2) Preparation process
[0104] Weigh cefadroxil passing through a 100-mesh sieve and mannitol, maltodextrin, sucrose, aspartame, menthol and sweet orange powder essence passing through a 80-mesh sieve, mix them evenly in a one-step granulator, and spray an appropriate amount of Granulate with water, weigh after drying, take 1% magnesium stearate, add it into a dry blender and mix with the dry granules evenly, compress the semi-finished product after the content is qualified, and the theoretical tablet weight is 550mg.
Embodiment 2
[0106] (1) Prescription
[0107] Ingredients 1000 pieces (g)
[0108] Cefadroxil 264g (equivalent to 250g cefadroxil anhydrous)
[0109]Mannitol 500g
[0110] Sucrose 246g
[0111] Maltodextrin 44g
[0112] Aspartame 2g
[0113] Sweet Orange Powder Flavor 30g
[0114] Menthol 2g
[0115] Appropriate amount of water
[0117] (2) Preparation process
[0118] 1) Cefadroxil, mannitol, maltodextrin, sucrose, aspartame and menthol are pulverized and sieved respectively;
[0119] 2) Mix the cefadroxil, mannitol, maltodextrin, sucrose, aspartame, menthol and sweet orange powder flavor that have been crushed and sieved in the previous step, and then spray an appropriate amount of water to granulate and dry to obtain dry granules ;
[0120] 3) Add magnesium stearate to the dry granules obtained in the upward step, mix evenly, and compress the semi-finished product to obtain final product.
Embodiment 3
[0122] (1) Prescription
[0123] Ingredients 1000 pieces (g)
[0124] Cefadroxil 528 parts by weight
[0125] Mannitol 240 parts by weight
[0126] 130 parts by weight of sucrose
[0127] Maltodextrin 88 parts by weight
[0128] Aspartame 4 parts by weight
[0129] 60 parts by weight of sweet orange powder essence
[0130] Menthol 4 parts by weight
[0131] Appropriate amount of water
[0132] 11 parts by weight of magnesium stearate.
[0133] (2) Preparation process
[0134] 1) Cefadroxil is crushed through a 100-mesh sieve, and mannitol, maltodextrin, sucrose, aspartame, and menthol are respectively crushed through a 80-mesh sieve;
[0135] 2) Weigh the cefadroxil, mannitol, maltodextrin, sucrose, aspartame, menthol and sweet orange powder essence that have been pulverized and sieved in the previous step according to the above dosage, and put them in a one-step granulator at a speed of 30r / min After mixing for 20 minutes under certain conditions, mix evenly, then s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average age | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com