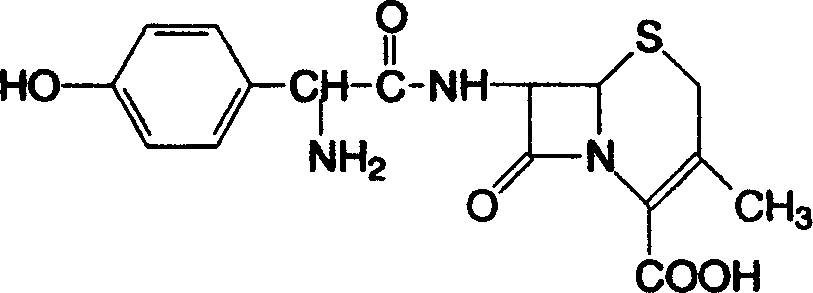
Cefadroxil oral disintegrant tablet, and its prepn. method
A technology of cefadroxil oral cavity and cefadroxil, which is applied in the field of cefadroxil orally disintegrating tablets and its preparation, can solve the problems of not being able to fully meet the medication needs of all patients, inconvenient taking of granules, and difficulty in swallowing medication for patients, etc. To achieve the effect of shortening the dissolution time, increasing the absorption point, and good compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Cefadroxil orally disintegrating tablet
[0047] components
weight
weight percentage
132g (equivalent to cefadroxine
Ampicillin anhydrate 125g)
93g
1.5g
44% (equivalent to cefadroxil
Ampicillin anhydrate 41.7%)
31%
0.5%
[0048] Menthol
5% Povidone 95% Ethanol
the solution
Low-substituted hydroxypropyl cellulose
gross weight
1g
6g
Appropriate amount
12g
48g
1.5g
1.5g
3g
300g
0.33%
4.55%
Appropriate amount
4%
16%
0.5%
0.5%
1%
A total of 1000 pieces were made
[0049] Concrete preparation method is as follows:
[0050] Weigh cefadroxil, mannitol, aspartame, menthol, sweet orange powder essence and ...
Embodiment 2
[0051] Embodiment 2: Cefadroxil orally disintegrating tablet
[0052] components
weight
weight percentage
Cefadroxil
50% ethanol
132g (equivalent to cefadroxine
Ampicillin anhydrate 125g)
180g
2.5g
1g
12g
Appropriate amount
26.4% (equivalent to cephalosporin
Amoxycillin anhydrous 25%)
60%
0.5%
0.2%
2.4%
Appropriate amount
Low-substituted hydroxypropyl cellulose
gross weight
60g
20g
80g
5g
2.5g
5g
500g
12%
4%
16%
1%
0.5%
1%
A total of 1000 pieces were made
[0054] Concrete preparation method is as follows:
[0055] Weigh cefadroxil, erythrose, aspartame, menthol, sweet orange powder essence and part of low...
Embodiment 3
[0056] Embodiment 3: Cefadroxil orally disintegrating tablet
[0057] components
weight
weight percentage
Cefadroxil
aspartame
50% ethanol
Crospovidone
264g (equivalent to cefadroxine
Ampicillin anhydrate 250g)
184g
3g
2g
12g
Appropriate amount
40g
80g
6g
44% (equivalent to cefadroxil
Ampicillin anhydrate 41.7%)
30.7%
0.5%
0.33%
2%
Appropriate amount
6.7%
13.3%
1%
[0058] sodium bicarbonate
gross weight
3g
6g
600g
0.5%
1%
A total of 1000 pieces were made
[0059] Concrete preparation method is as follows:
[0060] Weigh cefadroxil, erythrose, aspartame, menthol, sweet orange powder essence and part of cross-linked povidone that passed th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com