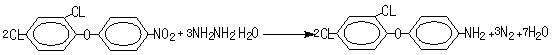Preparation method of 4-(2,4-dichlorophenoxy) aniline
A technology of dichlorophenoxy and dichlorophenyl, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., can solve the difficulty of complete separation of iron sludge and products, and poor extraction and washing production environment , iron slime can not deal with environmental pollution and other problems, to achieve the effect of low production cost, shortened production cycle, and increased synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Add 100ml of toluene and 50g of 2,4-dichlorophenyl-4'-nitrophenyl ether (purity 98%) in a 250ml four-necked reaction flask, start stirring to dissolve the material completely, add 2g of catalyst, and Add 16.2g of 80% hydrazine hydrate dropwise and fully react at 80°C. After the reaction is completed, filter the catalyst and recycle it. The filtrate is desolvated under reduced pressure to obtain 45.2g of 4-(2,4-dichlorophenoxy)aniline with a purity of 95%. , the yield is 98%.
Embodiment 2
[0012] Example 2: Add 100ml of xylene and 50g of 2,4-dichlorophenyl-4'-nitrophenyl ether (purity 98%) into a 250ml four-necked reaction flask, start stirring to dissolve the material completely, and add 2g of catalyst , and dropwise added 16.2g of 80% hydrazine hydrate, and fully reacted at 90°C. After the reaction was completed, the catalyst was filtered and recycled, and the filtrate was desolvated under reduced pressure to obtain 45.5g of 4-(2,4-dichlorophenoxy)aniline. 95.1%, yield 98.8%.
Embodiment 3
[0013] Example 3: Add 100ml of chlorobenzene and 50g of 2,4-dichlorophenyl-4'-nitrophenyl ether (purity 98%) into a 250ml four-necked reaction flask, start stirring to completely dissolve the material, add 2g of catalyst, And add 16.2g of 80% hydrazine hydrate dropwise, and fully react at 95°C. After the reaction is completed, the catalyst is filtered and recycled, and the filtrate is precipitated under reduced pressure to obtain 45g of 4-(2,4-dichlorophenoxy)aniline, with a purity of 95.3%. , yield 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com