A stable sodium folinate injection
A technology of sodium folinate and injection, applied in the directions of liquid delivery, emulsion delivery, antidote, etc., can solve the problem of not obtaining satisfactory results, and achieve the effect of reducing hydrolysis and oxidative decomposition, and preventing decomposition and deterioration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
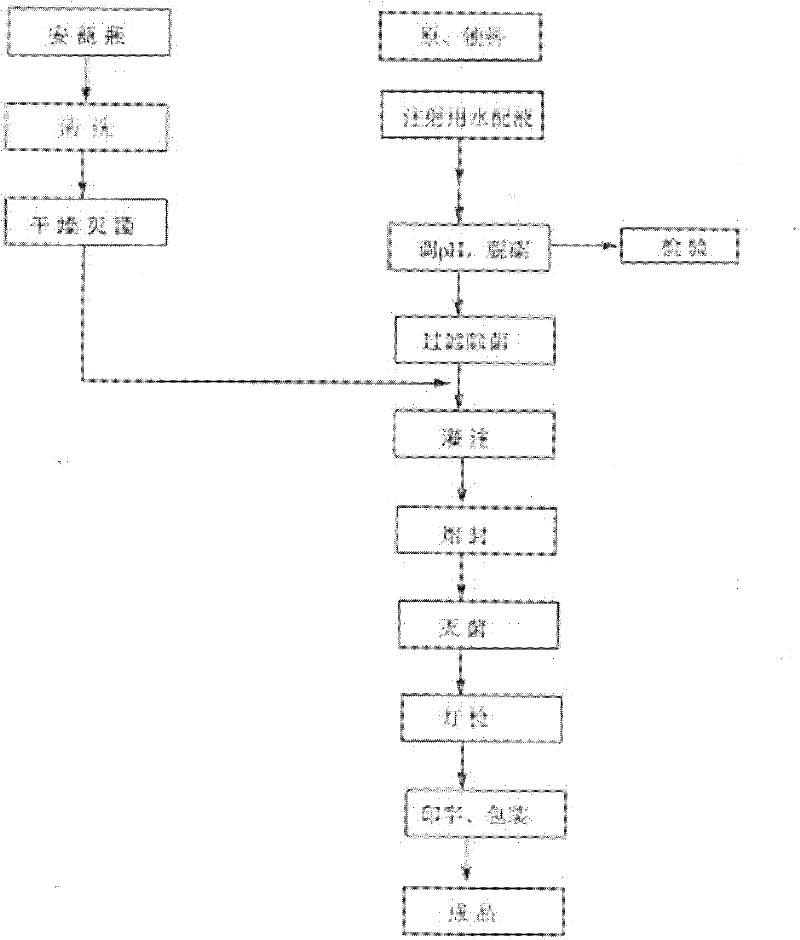
Image
Examples
Embodiment 1
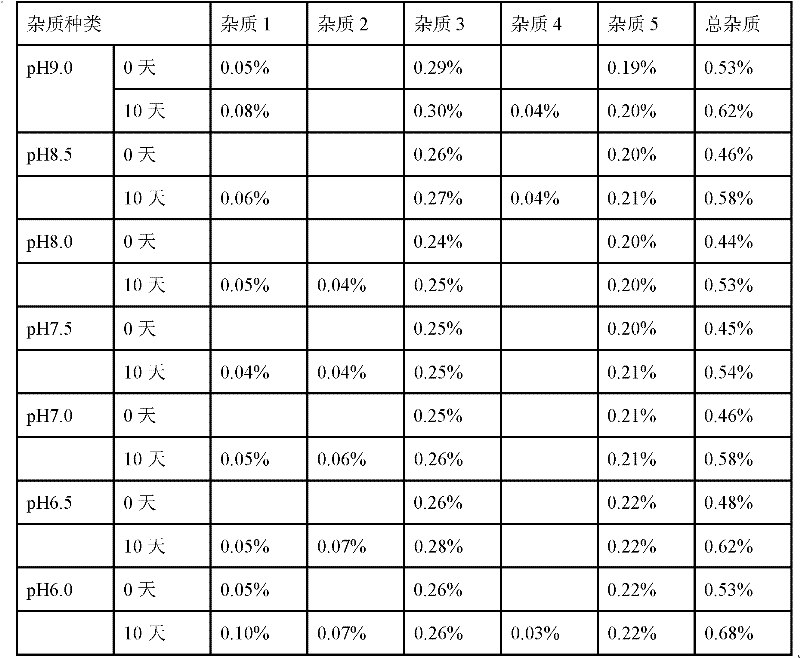
[0026] Example 1: Effect of pH on Stability
[0027] According to the method of the above preparation process, when adjusting the pH, adjust to different pH values in the following Table 1 to prepare the finished product, and the change of impurities after storage at 25°C for 10 days.
[0028]
[0029] Studies have shown that as the pH increases, the solubility of folinic acid increases. When the pH reaches 6.5, the drug is completely dissolved. When the pH is 7.8, it is the theoretical value for folinic acid to disodium folinate. Considering the reduction of blood vessel stimulation during injection, The pH should not be too high, and the above experiment also proves that the sodium folinate injection is relatively more stable when the pH is controlled between 6.5 and 8.5.
Embodiment 2
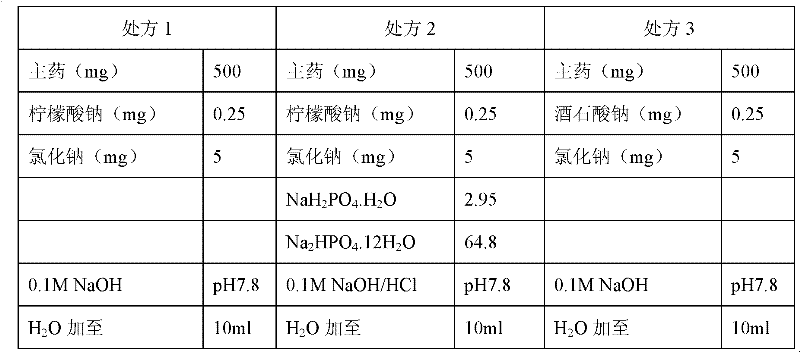
[0030] Embodiment 2: Sodium citrate is compared with other stabilizing agents on the influence of stability
[0031] The results are shown in the table below
[0032]
[0033] Prescription 1 used sodium citrate as a stabilizer, Prescription 2 added phosphate buffer saline, and Prescription 3 used tartaric acid instead of sodium citrate as a stabilizer.
[0034]
[0035] From the above results, after being placed at 25°C for 10 days, from the content point of view, they all decreased, and the degree of decline in prescription 1 and prescription 3 was relatively small. Therefore, it is judged that under its storage conditions (2-8°C), the change in content is acceptable.
Embodiment 3
[0036] Embodiment 3: the influence of the sodium citrate of different proportions on stability
[0037] The results are shown in the table below
[0038]
[0039] From the above results, the content ratio of the stabilizer sodium citrate to the main drug is in a linear relationship within a certain range of 0.0001: 1 to 5: 1, and the stability decreases with the reduction of the amount of sodium citrate, but without adding stabilizer When sodium citrate was added, the stability of the aqueous solution was even worse, which indicated that an appropriate amount of sodium citrate was very important for the long-term preservation of sodium folinate injection as a stabilizer. Especially when the weight ratio between the main ingredient and sodium citrate is 1:0.0005, the stability is the best.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com