Preparation process of 2-ethyl hexenal and 2-ethyl hexanol
A preparation technology of ethylhexenal and 2-ethylhexenal, which is applied in the field of preparation technology of 2-ethylhexenal and 2-ethylhexanol, can solve the problems of complex process, high energy consumption, side reactions, etc., and achieve simplification Process, increase the concentration of alkali, the effect of mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
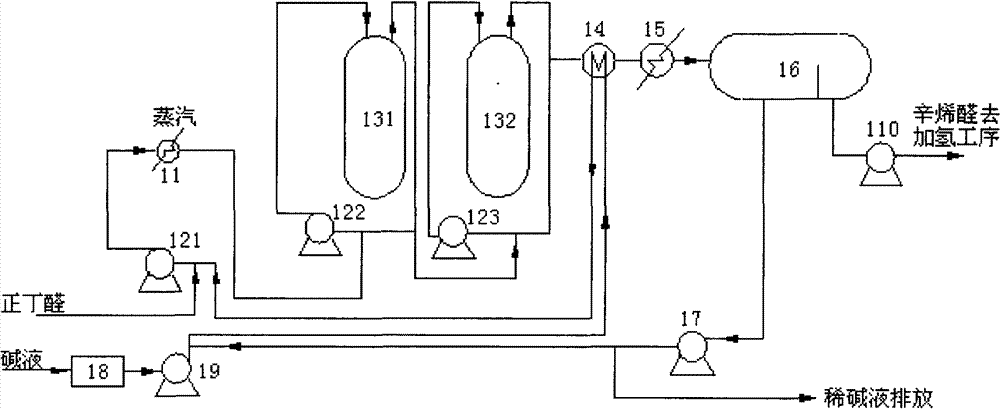
Embodiment 1
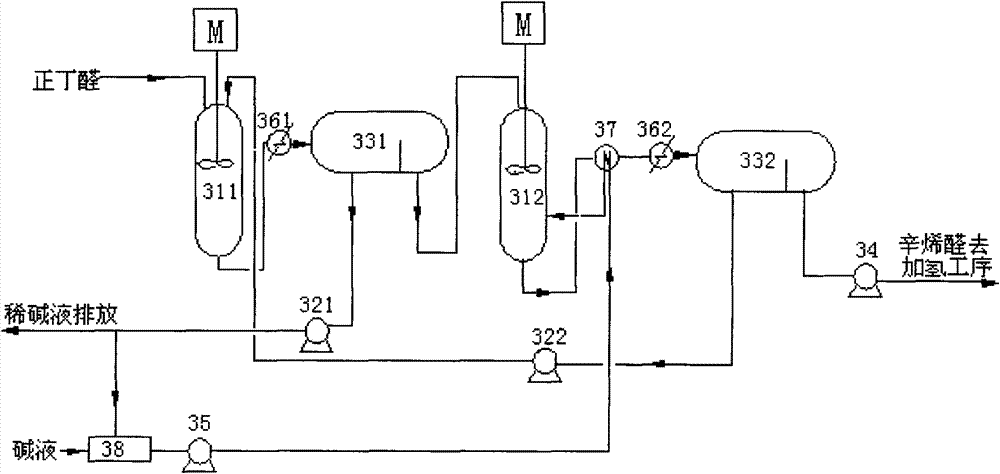
[0041] like image 3 As shown, a system for preparing 2-ethylhexenal provided by the present invention includes a first reactor 311, a second reactor 312, a first chromatograph 331 and a second chromatograph 332; wherein , the outlet of the first reactor 311 is connected to the inlet of the first chromatograph 331 through a heat exchanger 361 through a pipeline; the lower layer outlet of the first chromatograph 331 communicates with the outside world through the lye circulation pump 321, and the first chromatograph The upper floor outlet of 331 is connected with the inlet of the second reactor 312 through a pipeline; the lye inlet of the second reactor 312 is connected with the lye tank 38 through the lye supply pump 35, and the outlet of the second reactor 312 is connected with the second The inlet of chromatograph 332 is connected through pipeline through heat exchanger 362; The outlet of the upper layer is connected to the downstream process through the pipeline through th...
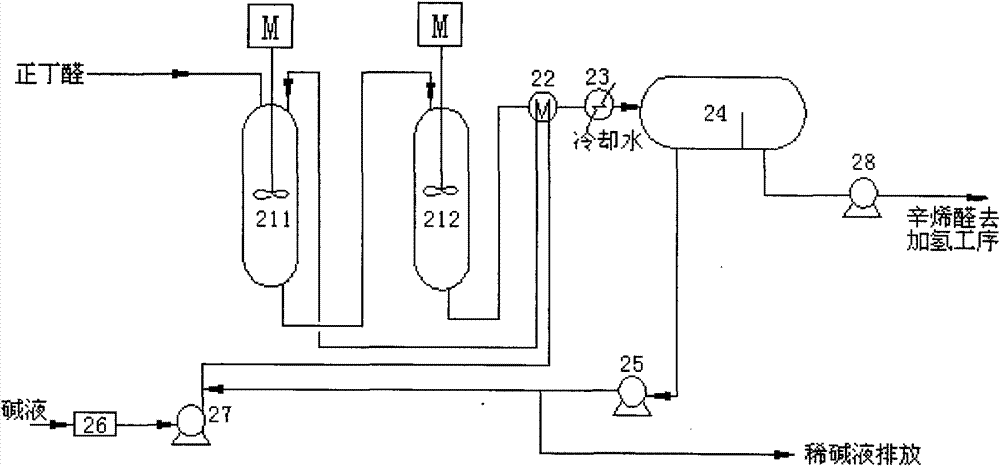
Embodiment 2
[0046] The process flow is attached image 3 , Fresh raw material 100ml / min containing 99.8wt% n-butyraldehyde enters the first condensation reactor 311 with the alkaline catalyst aqueous solution 31.2ml / min from the lye circulating pump 322, mixes and reacts, and the reaction temperature is 80°C. The residence time is 15 minutes. After the discharge from the first condensation reactor 311 is cooled to 40°C by the cooler 361, it is sent to the chromatograph 331 for stratification. Reprocessing, upper layer butyraldehyde and octenal mixed solution (56.4wt% of 2-ethylhexenal content), is sent in the second condensation reactor 312 with 85ml / min, with the 47ml from lye supply pump 5 After the 2wt% lye of / min is mixed, react further, reaction temperature 120 ℃, residence time 10min, the output of the second condensation reactor 312 is sent into chromatograph 332 after cooler 362 is cooled to 40 ℃, through layer Impurities such as 2-ethylhexenal 97wt% and polybutyraldehyde 0.7%, ...
Embodiment 3
[0048] The process flow and reaction conditions are the same as in Example 2, except that the raw materials are fresh 99wt% n-butyraldehyde and 1wt% isobutyraldehyde. After the first condensation reactor 311 completes the reaction, the chromatograph 331 is layered, and the upper layer butyraldehyde and octenal mixed solution (the content of 2-ethylhexenal is 53.1wt%) is reacted 322 through the second condensation reactor. , separated by chromatography 332 to obtain the upper layer solution is 96.4wt% of 2-ethylhexenal and 0.7% of polybutyraldehyde, 0.17% of 2-ethyl-4-methylpentenal and unreacted n-butyraldehyde 1% and other impurities. After hydrogenation and rectification, the final product of 2-ethylhexanol with a purity of ≥99.6% is obtained. The main impurities of the product are 0.14% of 2-ethyl-4-methylpentanol, 0.12% of water, and 2-ethylhexanol Aldehyde 0.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com