A kind of chalcone derivative, its preparation method and its application as a tumor cell apoptosis inducer
A technology of chalcone derivatives and derivatives, applied in the field of chalcone and its preparation, can solve the problem that chalcone derivatives do not have the effect of tumor cell apoptosis inducer, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
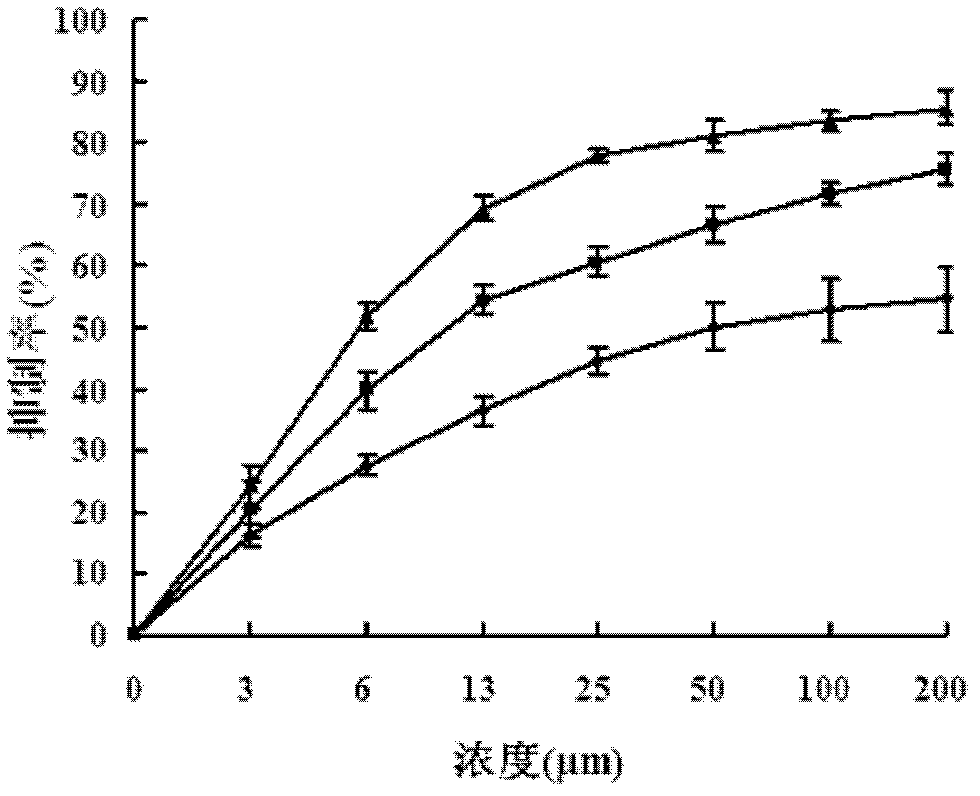
[0018] Specific Embodiment 1: This embodiment provides a chalcone derivative whose chemical name is 2′,3-dihydroxy-4,4′,5′,6′-tetramethoxychalcone and whose molecular formula is :
[0019]
[0020] Me described in the molecular formula is a methyl group.
specific Embodiment approach 2
[0021] Specific embodiment 2: This embodiment provides a preparation method of chalcone derivatives, the chemical name is 2′, 3-dihydroxy-4,4′, 5′, 6′-tetramethoxychalcone is Done as follows:
[0022] 1. Synthesis of 2,6-dimethoxybenzoquinone: K 3 Fe(CN) 6 Dissolve in water, then add 1,3,5-trimethylbenzene and acetone, stir at room temperature, and every three hours, add hydrogen peroxide with a mass fraction of 30%, stir for 15 hours, and precipitate a yellow-green solid; The separated yellow-green solid is decolorized with methanol to obtain yellow solid 2,6-dimethoxybenzoquinone at last; the K described in step one 3 Fe(CN) 6The mass ratio with water is (0.8~1.2): 5, the mass ratio of the 1,3,5-trimethylbenzene and water added is (1.6~1.75): 1, the acetone added and water The mass ratio is (9.5~10.5): 1, and the mass ratio of the hydrogen peroxide and water added with a mass fraction of 30% is (3~4): 1;
[0023] 2. Synthesis of 2,6-dimethoxy-p-hydroxybenzene: 2,6-dimet...
specific Embodiment approach 3
[0036] Specific embodiment three: the difference between this embodiment and specific embodiment two: K described in step one 3 Fe(CN) 6 The mass ratio with water is 1:5, the mass ratio of the added 1,3,5-trimethylbenzene and water is 1.68:1, and the mass ratio of the added acetone and water is 10:1, so The mass ratio of the added mass fraction of 30% hydrogen peroxide to water is 3.4:1. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com