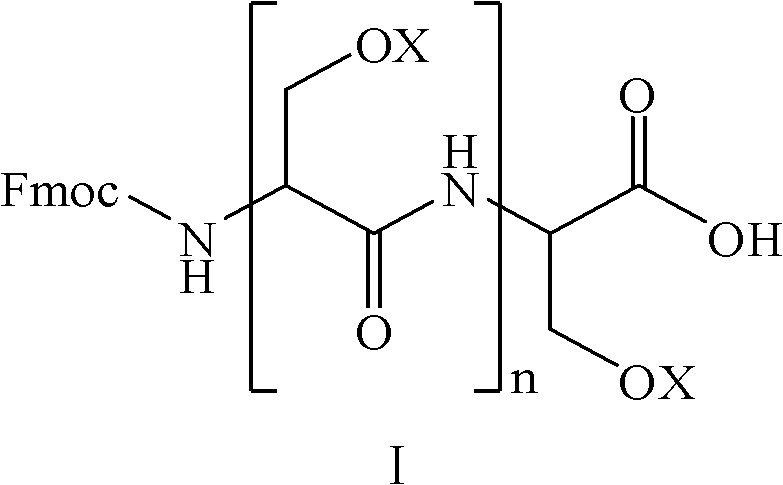
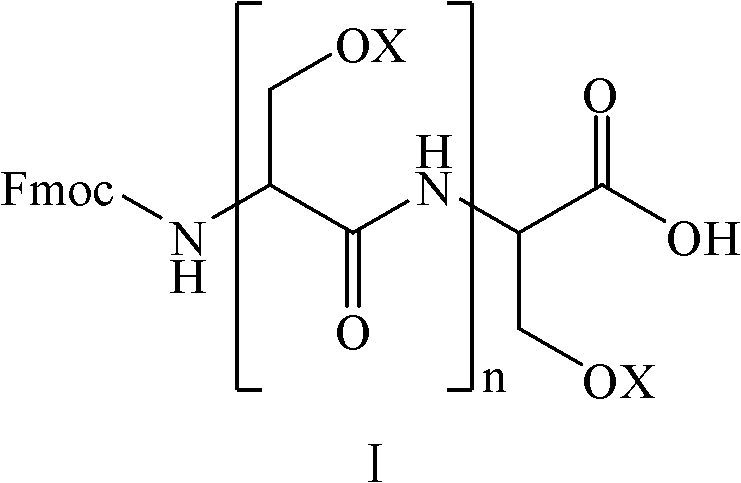
Amino-protecting serine oligopeptide as well as preparation method and application thereof
An amino-protected and serine technology, applied in the field of amino acid modification compounds, can solve the problem of no amino-protected serine oligopeptide, etc., and achieve the effects of improving yield and purity and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1Fmoc-Ser(tBu)-Ser(tBu)-Ser(tBu)-Ser(tBu)-OH
[0024] Take 3.0mol Ser(tBu) and 3.0mol HOBt, and dissolve them with an appropriate amount of DMF; take another 3.0mol DIC (N,N-diisopropylcarbodiimide), slowly add it to the protected amino acid DMF solution under stirring, and Stir the reaction at room temperature for 30 minutes to obtain the activated protected amino acid solution.
[0025] Take 1Kg of Fmoc-Ser(tBu)-2-Cl-Trt-resin (substitution value is 1.0mmol / g); use 6L PIP / DMF solution to deprotect for 25 minutes, wherein the volume concentration of PIP is 20%. After filtering, the resin was washed 3 times with MDF and DCM respectively, and the above-mentioned protected amino acid solution was added, and the reaction was stirred at room temperature for 3 hours. After the reaction was completed, the filtered resin was washed 3 times with MDF and DCM respectively to obtain Fmoc-Ser(tBu)-Ser( tBu)-OH.
[0026] The above reaction was repeated ...
Embodiment 2
[0028] Example 2 Application of Fmoc-Ser(tBu)-Ser(tBu)-Ser(tBu)-Ser(tBu)-OH in Polypeptide Synthesis
[0029] Take Fmoc-Lys(Boc)-2-Cl-Trt-resin, after Fmoc deprotection, couple with Fmoc-Ser(tBu)-Ser(tBu)-Ser(tBu)-Ser(tBu)-OH to prepare 5-peptide resin was obtained.
[0030] The coupling method is specifically:
[0031] 1. Activation of Fmoc-Ser(tBu)-Ser(tBu)-Ser(tBu)-Ser(tBu)-OH:
[0032] Take 0.3mol Fmoc-Ser(tBu)-Ser(tBu)-Ser(tBu)-Ser(tBu)-OH and 0.3mol HOBt, and dissolve them with an appropriate amount of DMF; take another 0.3mol DIC, slowly add to the aforementioned DMF under stirring solution, stirred and reacted at room temperature for 30 minutes to obtain an activated protected amino acid solution.
[0033] 2. De-Fmoc protection of Fmoc-Lys(Boc)-2-Cl-Trt-resin:
[0034] Take 200 g of Fmoc-Lys(Boc)-2-Cl-Trt-resin (substitution value: 0.5 mmol / g), use 1 L of 20% PIP / DMF solution to deprotect for 25 minutes, filter and wash with DMF for 5 times to obtain de-Fmoc resin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com