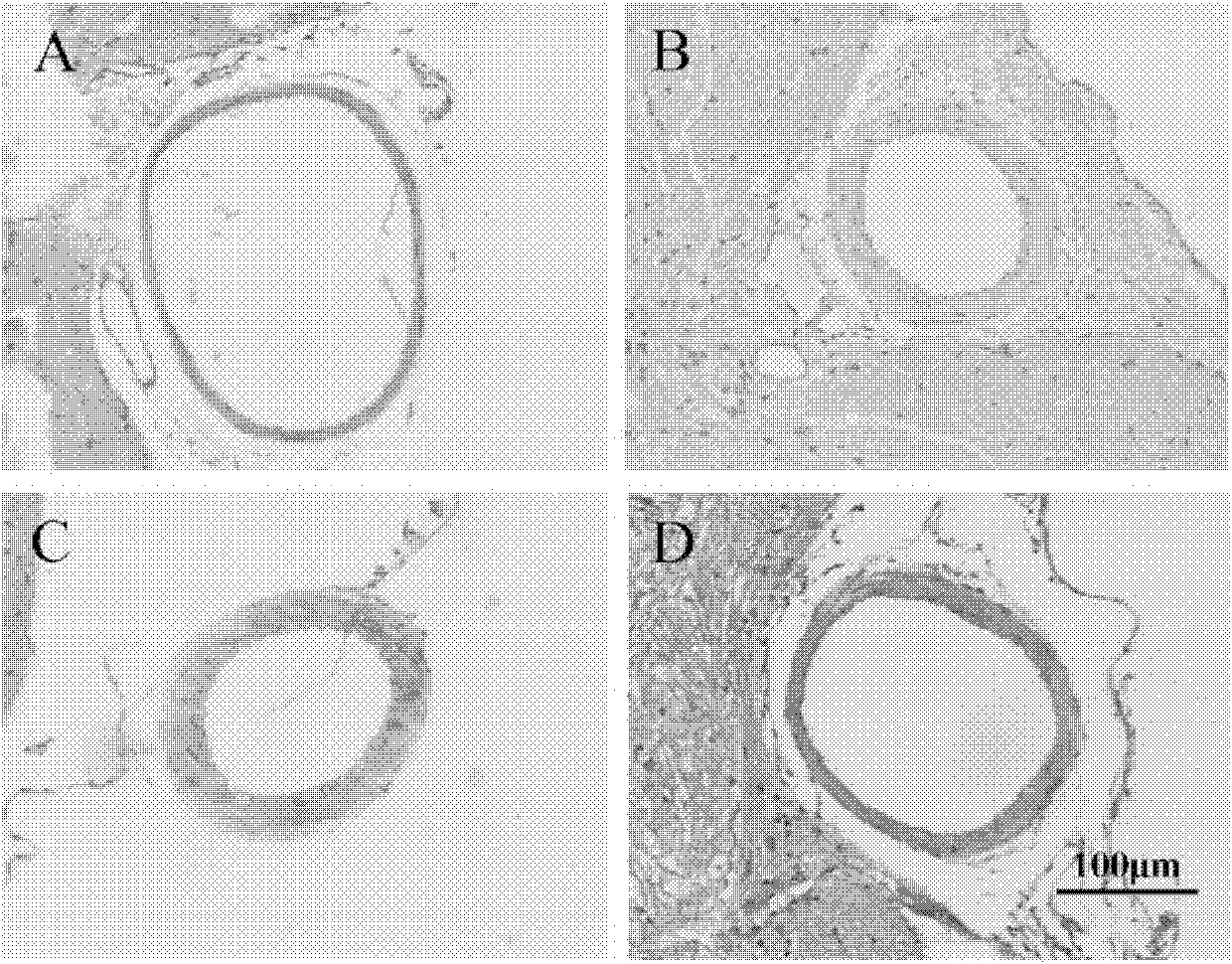
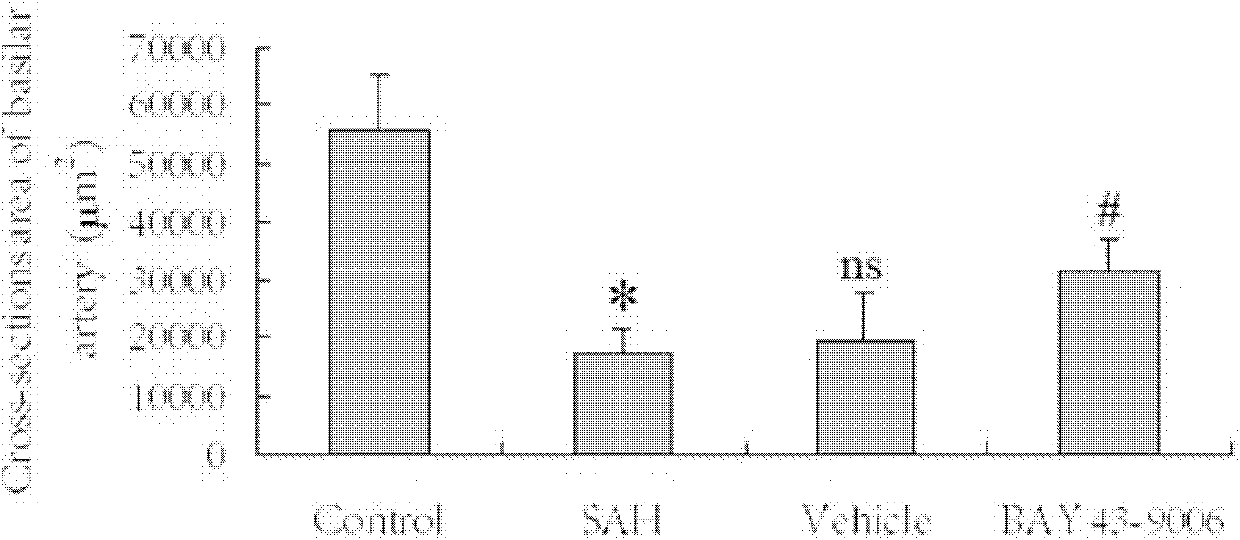
Application of sorafenib to treatment of cerebral vasospasm (CVS) after subarachnoid hemorrhage (SAH)
A technology for cerebral vasospasm and subarachnoid space, which can be used in cardiovascular system diseases, drug combinations, active ingredients of heterocyclic compounds, etc., can solve the problems of reduced bioavailability of sorafenib, and achieve the effect of preventing and treating cerebral vasospasm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Effect of BAY 43-9006 on cerebral vasospasm after SAH
[0040] Materials and methods
[0041] 1. Materials
[0042] 1. Main reagents and medicines
[0043] Trizol total RNA extraction reagent was purchased from U.S. Invitrogen; diethyl pyrocarbonate (DEPC) was purchased from U.S. Sigma; M-MLV reverse transcriptase was purchased from U.S. Promega; Taq DNA polymerase was purchased from U.S. New England Biolabs; dNTPs were purchased from NewEngland Biolabs, USA; six random primers were purchased from Promega, USA; SYBR Green I was purchased from Takara, Japan; chloroform was purchased from Shanghai Chemical Reagent Company; isopropanol was purchased from Shanghai Chemical Reagent Company; absolute ethanol was purchased from Shanghai Chemical Reagent Company.
[0044] Rabbit anti-phospho-ERK1 / 2 (Thr202 / Tyr204) antibody was purchased from US cell signaling company; rabbit anti-IkB-α (C-21) was purchased from US Santa Cruz company.
[0045] PCR primers: The cDNA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com