Anti-HIV (Human Immunodeficiency Virus) infection polypeptide, composition and application
A technology of derivatives and peptide sequences, applied in the field of acquired immunodeficiency syndrome, treatment or prevention of related diseases caused by HIV infection, non-physiological toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: the preparation of compound 1
[0115] A standard Fmoc solid-phase peptide synthesis method was used. All peptide sequences are amidated at the C-terminus and acetylated at the N-terminus. Rink Amide resin is selected, and the peptide chain is extended from the C-terminus to the N-terminus. The condensing agent is HBTU / HOBt / DIEA. The deprotecting agent is piperidine / DMF solution. The lysing agent is trifluoroacetic acid (TFA), and the crude peptide is dissolved in water and then freeze-dried for storage. Separation and purification are carried out by medium-pressure liquid chromatography or high-pressure liquid chromatography (HPLC), and the content of pure peptide is >90%. The molecular weight of the peptide sequence was determined by matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS).
[0116] The microwave peptide synthesis conditions are as follows:
[0117] Amino acids: 0.2M DMF solution,
[0118] Activator: 0.45M ...
Embodiment 2-34
[0127] Embodiment 2-34: Preparation of compound 2-34
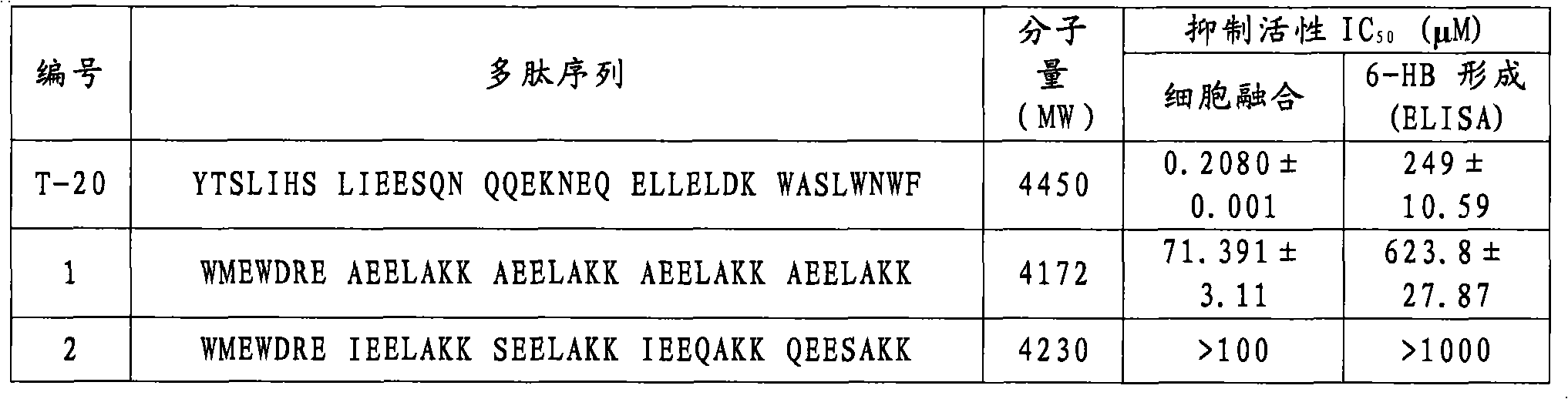
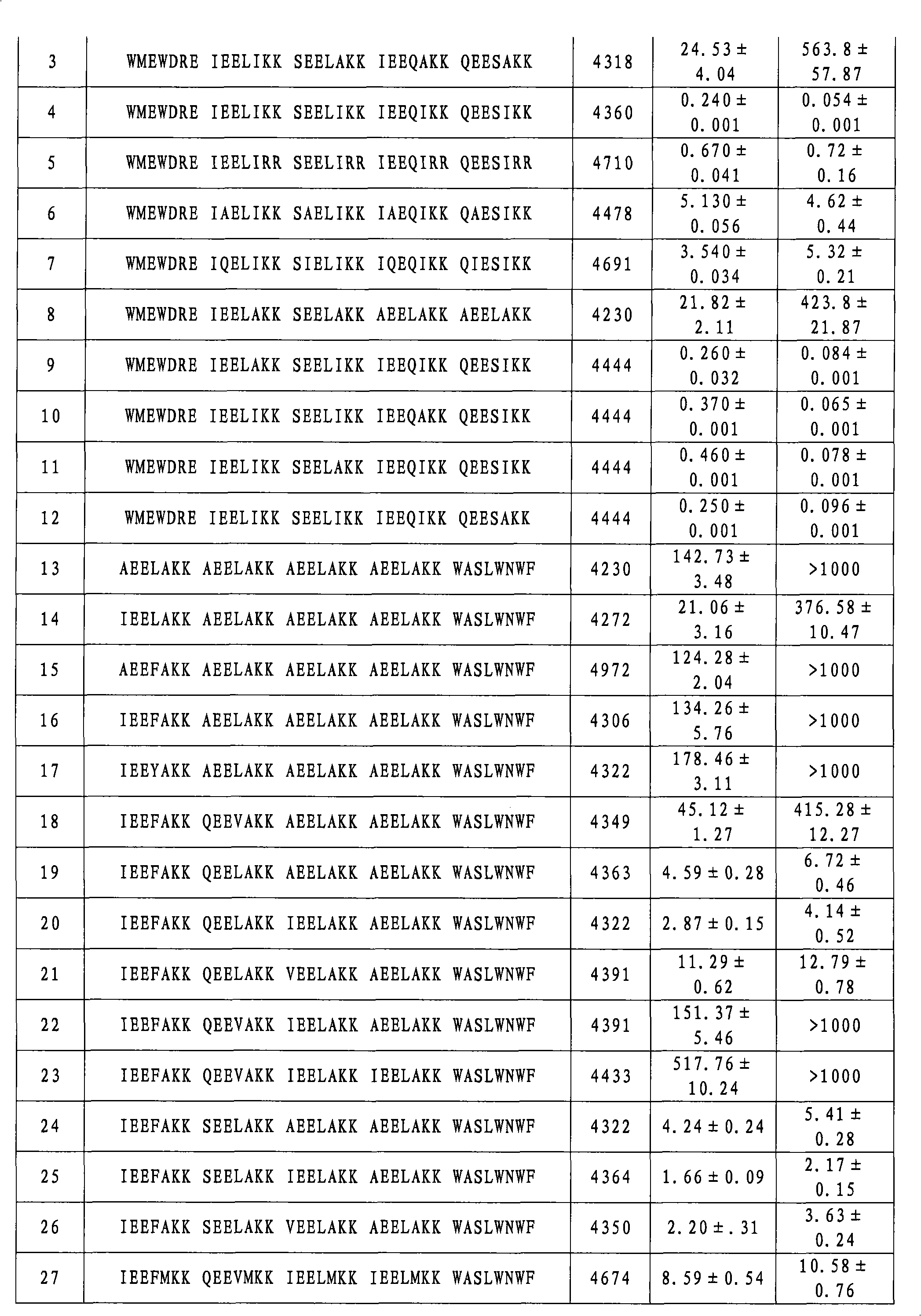
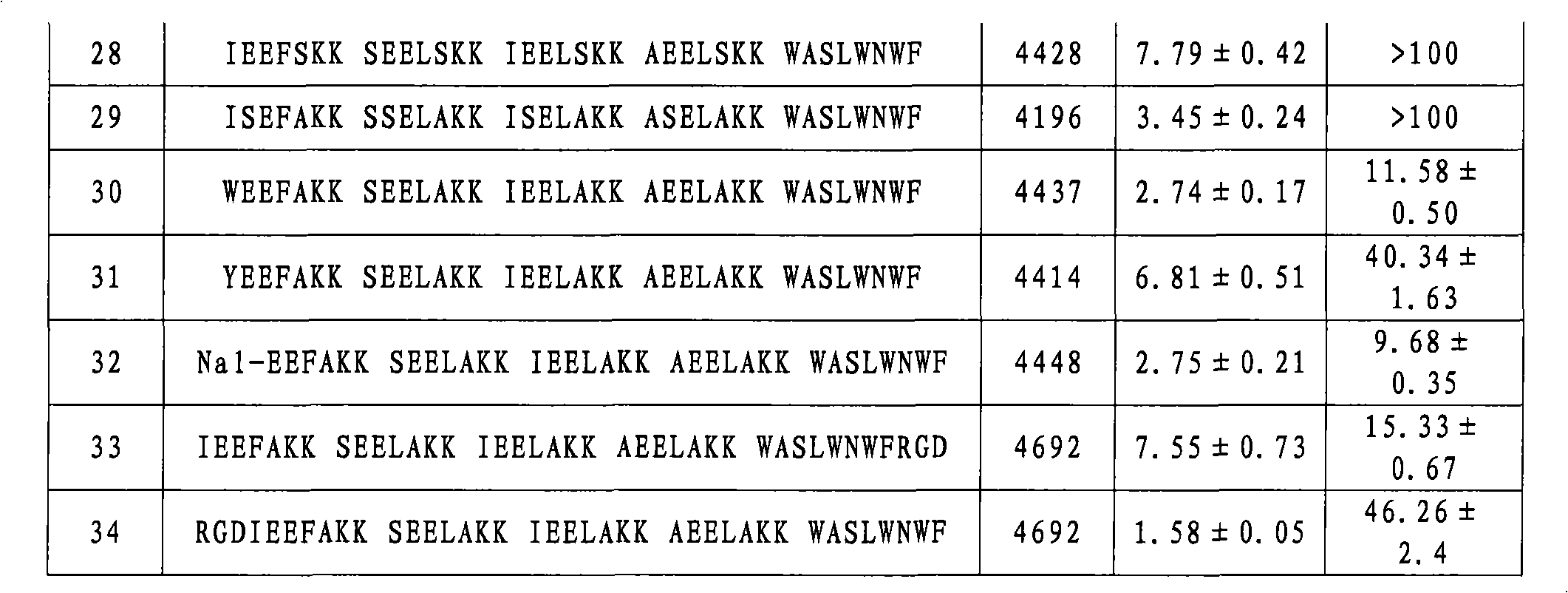
[0128] Compounds No. 2-No. 34 were synthesized according to the method in Example (1), except that the corresponding amino acid residues were replaced. The molecular weights of compounds 2-34 are shown in Table 1 below.
Embodiment 35
[0129] Example 35: Detection of inhibition of HIV-1 biological activity
[0130] 1) Evaluation of compounds inhibiting HIV-1-mediated cell-cell fusion activity (IC 50 )
[0131] Detection of HIV-1-Mediated Cell-Cell Fusion by Stain Transfer Assay: HIV-1 IIIB Infected H9 cells (H9 / HIV-1 IIIB ) was labeled with a fluorescent reagent Calcein-AM (Molecular Probes, Inc., Eugene, OR), and then in a 96-well plate at 37 ° C with or without the test compound and MT-2 cells (ratio = 1:10) Cultivate for 2h. Test compounds were serially diluted two-fold from a concentration of 250 μg / ml. Fused and unfused Calcein-labeled HIV-1 infected cells were counted using an inverted fluorescent microscope (Zeiss, Germany). Calculation IC 50 value.
[0132] 2) ELISA detection compound inhibits gp41 6-HB formation activity (IC 50 )
[0133] Sandwich ELISA method to detect C-peptide inhibition of gp41 6-HB formation activity: the test compound was serially diluted two-fold from the concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com