Synthesis method of natural equivalent anise alcohol
A synthetic method and equivalent technology, applied in the field of synthesis of anisyl alcohol, can solve the problems of surrounding environmental pollution, insufficient yield, low total yield, etc., and achieve the effects of cost saving, pure aroma, and innovative process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
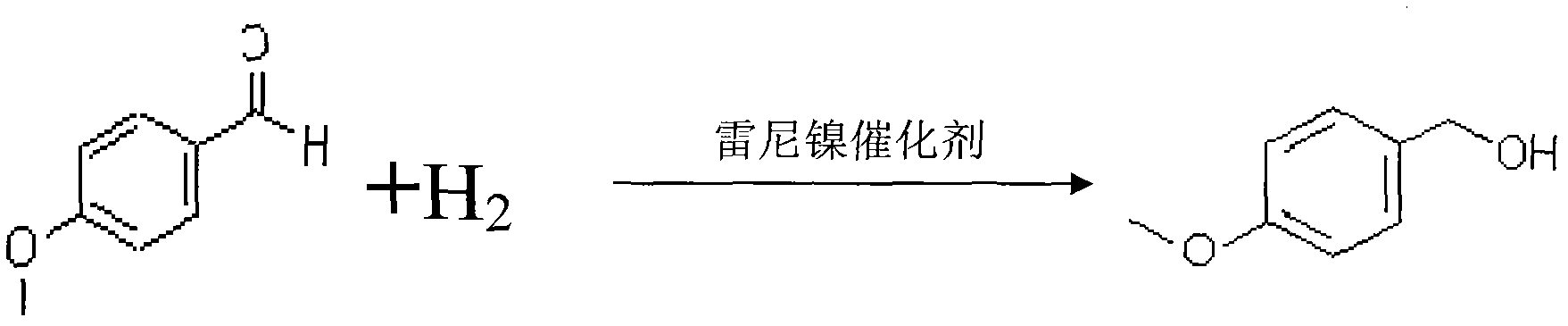
[0022] Weigh 250 grams of natural equivalent anisaldehyde, put it into the reactor, then weigh 1 gram of Raney nickel catalyst and dissolve it in 50 grams of ethanol, and put it into the reactor. Start the stirring device to stir to make the reactants mix evenly, and then inject hydrogen until the pressure is 0.15MPa, while observing the change of pressure and temperature, stop injecting hydrogen after the pressure stabilizes, and keep the temperature at about 25-30°C. After 6 hours, slowly raise the temperature to 58-60°C, keep the temperature for 32 hours, pay attention to the change of hydrogen pressure during this period, and replenish hydrogen in time to make the hydrogenation reaction stable, until no more hydrogen is absorbed, that is, the hydrogen pressure does not change. until. After the hydrogenation reaction is completed, the semi-finished liquid anisyl alcohol is discharged. Slowly open the discharge valve of the reaction kettle, the feed liquid is discharged und...
Embodiment 2
[0025] Weigh 250 grams of natural equivalent anisaldehyde, put it into the reactor, then weigh 0.95 grams of Raney nickel catalyst, dissolve it in 50 grams of ethanol, and put it into the reactor. Start the stirring device to stir, so that the reactants are evenly mixed, inject hydrogen until the pressure is 0.12MPa, observe the changes in pressure and temperature, stop injecting hydrogen after the pressure stabilizes, and keep the temperature at about 25-30°C, After 6 hours, slowly raise the temperature to 55-60°C, and keep it warm for 33 hours. During this period, pay attention to the change of hydrogen pressure, and replenish hydrogen in time to make the hydrogenation reaction stable, until no more hydrogen is absorbed, that is, the hydrogen pressure does not change. until. After the hydrogenation reaction is completed, the semi-finished liquid anisyl alcohol is discharged. Slowly open the discharge valve of the reaction kettle, the feed liquid is discharged under the acti...
Embodiment 3
[0028] Weigh 250 grams of natural equivalent anisaldehyde, put it into the reactor, then weigh 1.03 grams of Raney nickel catalyst, dissolve it in 49 grams of ethanol, and put it into the reactor. Start the stirring device to stir, so that the reactants are mixed evenly, and the hydrogen gas is introduced until the pressure is 0.12-0.15 MPa. While observing the changes in pressure and temperature, stop the hydrogen gas injection after the pressure stabilizes, and keep the temperature at 26-30 °C After about 6 hours, slowly increase the temperature to 55-58°C, and keep the temperature for 31 hours. During this period, pay attention to the change of hydrogen pressure, and replenish hydrogen in time to make the hydrogenation reaction proceed stably until no more hydrogen is absorbed, that is, the hydrogen pressure does not increase. until it changes. After the hydrogenation reaction is completed, the semi-finished liquid anisyl alcohol is discharged. Slowly open the discharge va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com