Method for preparing 17alpha-hydroxyl progesterone
The technology of hydroxyprogesterone and hydroxyl group is applied in the field of preparation of 17α-hydroxyprogesterone, which can solve the problems of low yield and the like, and achieve the effects of short route, high safety and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
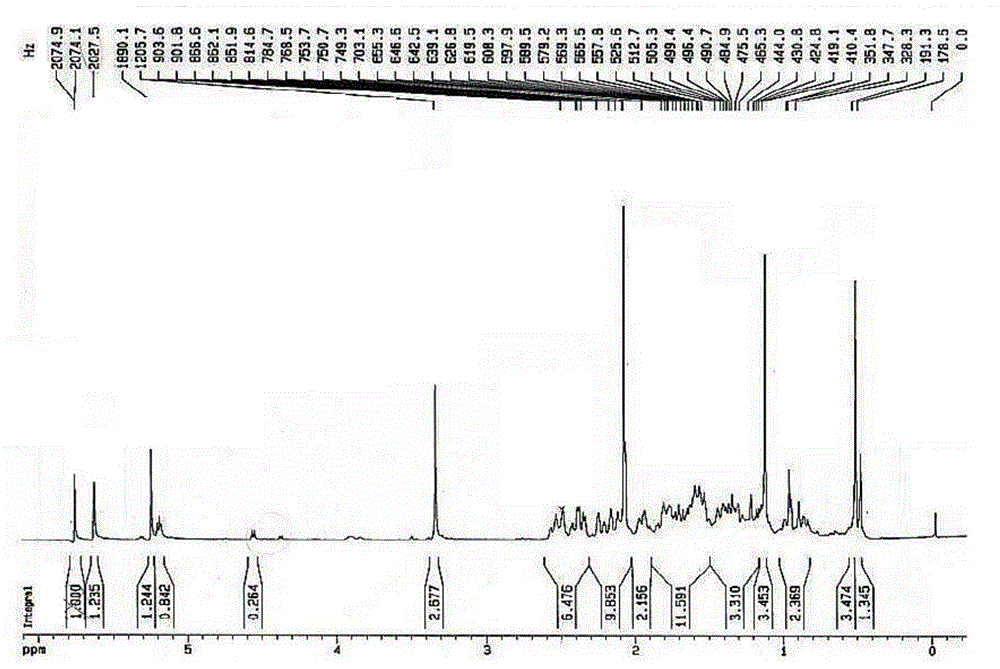
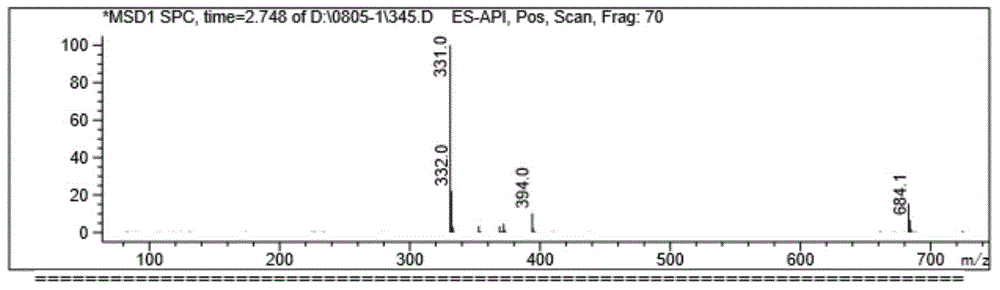
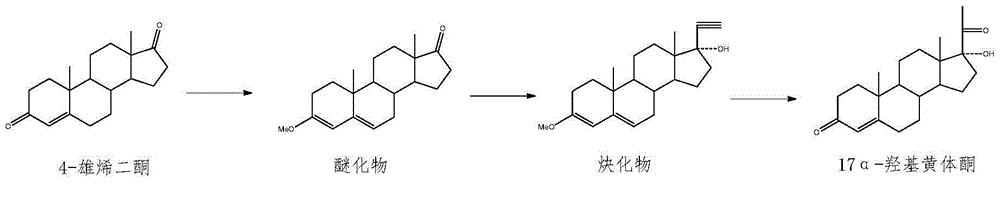
[0027] Anhydrous methanol (25.0mL) and 4-AD (11.50g) were dropped into the dried reactor (the reactor was blown dry with nitrogen) and stirred, trimethyl orthoformate (6.80g) and PTS (0.187g) were added, and the temperature was 40° C. Stir and keep for 4h, sampling point plate [developing solvent=ethyl acetate / petroleum ether (EA / PE)=2.5 / 1] without raw material, then drop to room temperature, add triethylamine to neutralize pH=7, cool down to -5°C, Stir for 1 h, filter, rinse with an appropriate amount of cold methanol and dry to obtain 3-methoxyandrost-3,5-dien-17 ketone.
[0028] Potassium hydroxide and calcium carbide were ground into powders with a mixer respectively, and sieved through a 50-mesh sieve for use. Potassium hydroxide (7.75g), calcium carbide (21.00g), tert-butanol (24.5mL) and ethylenediamine (65.0 mL) was added to a 500g reaction flask, kept at 40°C for 16h, cooled to 25°C, added 3-methoxyandrost-3,5-dien-17 ketone (11.80g), stirred for 5h, and slowly added ...
Embodiment 2
[0034] Anhydrous ethanol (25.0mL) and 4-AD (11.00g) were dropped into the dried reactor (the reactor was blown dry with nitrogen) and stirred, and triethyl orthoformate (7.80g) and PTS (0.187g) were added at 40° C. Stir and hold for 4h, the sampling point plate (developer=EA / PE=2.5 / 1) is free of raw materials and then cooled to room temperature, add triethylamine to neutralize pH=7, cool down to -5°C, stir for 1h, filter, and cool methanol appropriately Rinse and dry to obtain 3-ethoxyandrost-3,5-dien-17 ketone.
[0035] Potassium hydroxide and calcium carbide were ground into powders with a mixer, respectively, and passed through an 80-mesh sieve for use. Potassium hydroxide (7.75g), calcium carbide (21.00g), tert-butanol (24.5mL) and ethylenediamine (65.0 mL) into a 500g reaction flask, kept at 40°C for 16h, cooled to 25°C, added 3-ethoxyandrost-3,5-dien-17 ketone (11.80g), stirred for 5h, and slowly added dropwise under nitrogen protection. Acetic acid (54.5mL), water (300...
Embodiment 3
[0037] Anhydrous methanol (25.0mL) and 4-AD (10.00g) were put into the dried reaction kettle (the reaction kettle was blown dry with nitrogen) and stirred, trimethyl orthoformate (6.80g) and PTS (0.187g) were added, and the temperature was 40° C. Stir and hold for 4h, the sampling point plate (developer=EA / PE=2.5 / 1) is free of raw materials and then cooled to room temperature, add triethylamine to neutralize pH=7, cool down to -5°C, stir for 1h, filter, and cool methanol appropriately Rinse and dry to obtain 3-methoxyandrost-3,5-dien-17 ketone.
[0038] Grind sodium hydroxide and calcium carbide into powders with a mixer, respectively, and pass through a 50-mesh sieve for later use. Mix sodium hydroxide (5.85g), calcium carbide (21.00g), propanol (19.6mL) and diethylamine (70.0mL) ) into a 500g reaction flask, kept at 40°C for 16h, cooled to 25°C, added 3-methoxyandrost-3,5-dien-17 ketone and stirred for 5h, under nitrogen protection, slowly added dropwise acetic acid, water, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com