Dibenzothiophene-based aromatic phosphine oxide compound and preparation method and application thereof
A technology of dibenzothiophene and diphenylphosphine oxide, which is applied in the field of dibenzothiophene aromatic phosphine oxide and its preparation and application, can solve the problems of low efficiency and brightness, poor efficiency stability, and driving voltage of electrophosphorescent devices. Advanced problems, to achieve a wide range of application prospects, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
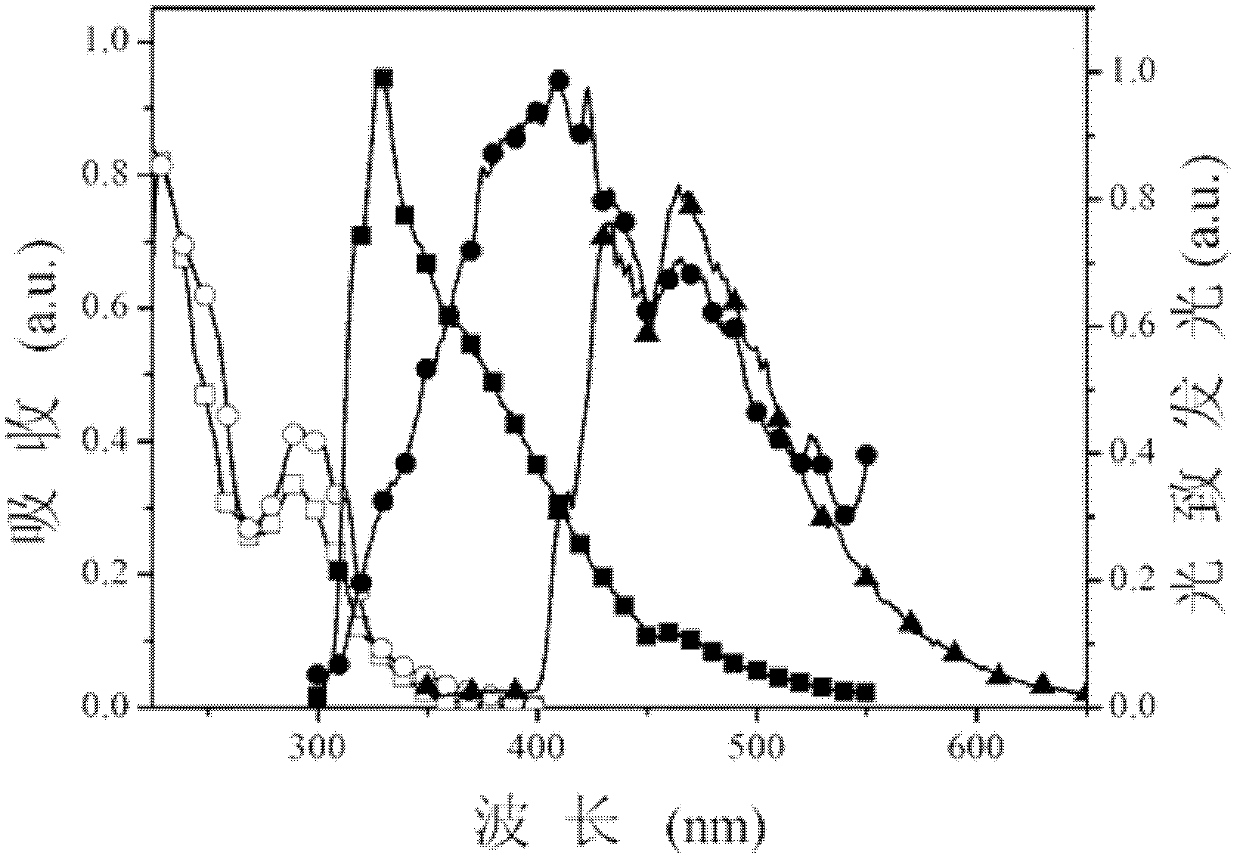
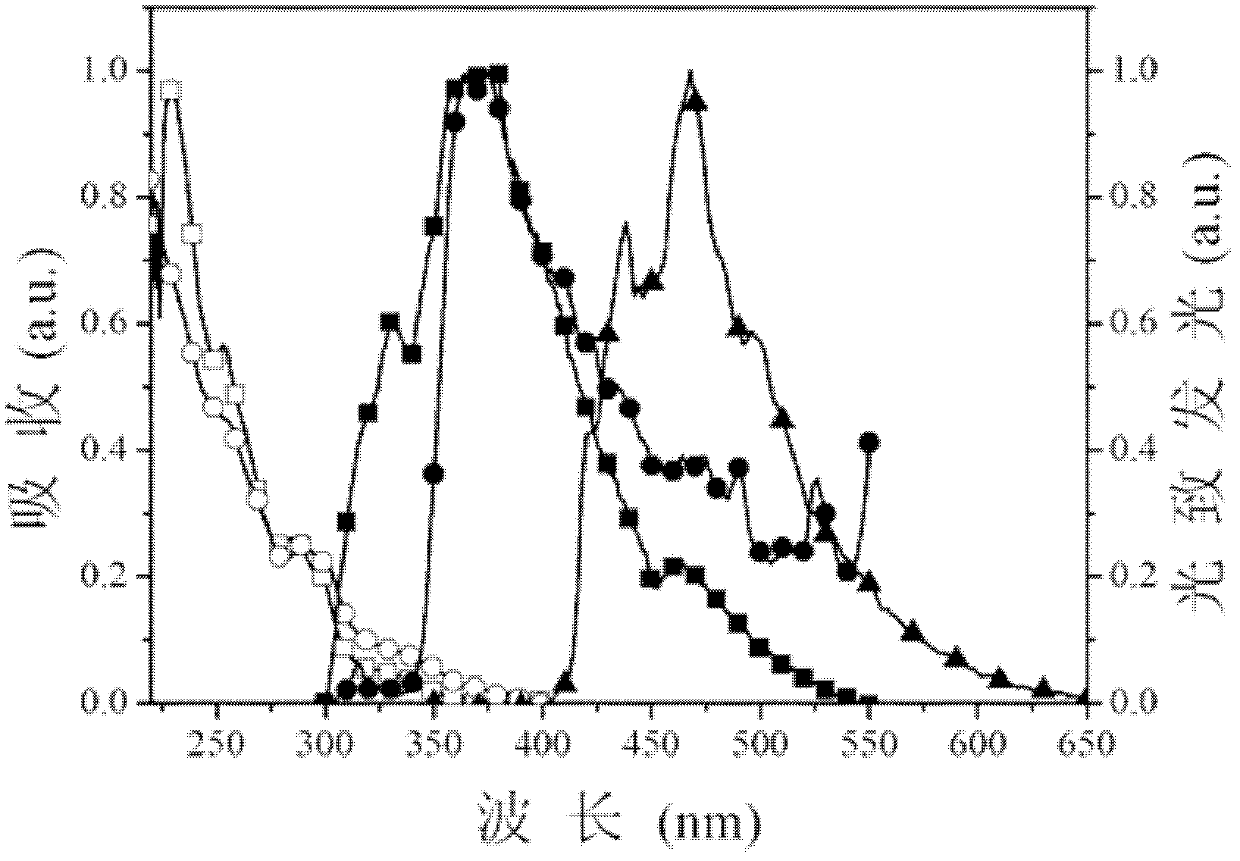
[0034] Specific embodiment 1: This embodiment is a dibenzothiophene aromatic phosphine oxide compound, which is based on dibenzothiophene, and the diphenylphosphine group is at the 4-position or 4,6-position of dibenzothiophene Substituted obtained, its structural general formula is as follows:
[0035] Wherein, X is H or a diphenylphosphine group.
[0036] The dibenzothiophene aromatic phosphine oxide compound of this embodiment is based on dibenzothiophene, and in the presence of tetramethylethylenediamine (TMEDA), the 4-position or 4,6-position of dibenzothiophene occurs successively Lithiation, phosphination and oxidation reactions can give aromatic phosphine oxides substituted at 4 or 4,6 positions.
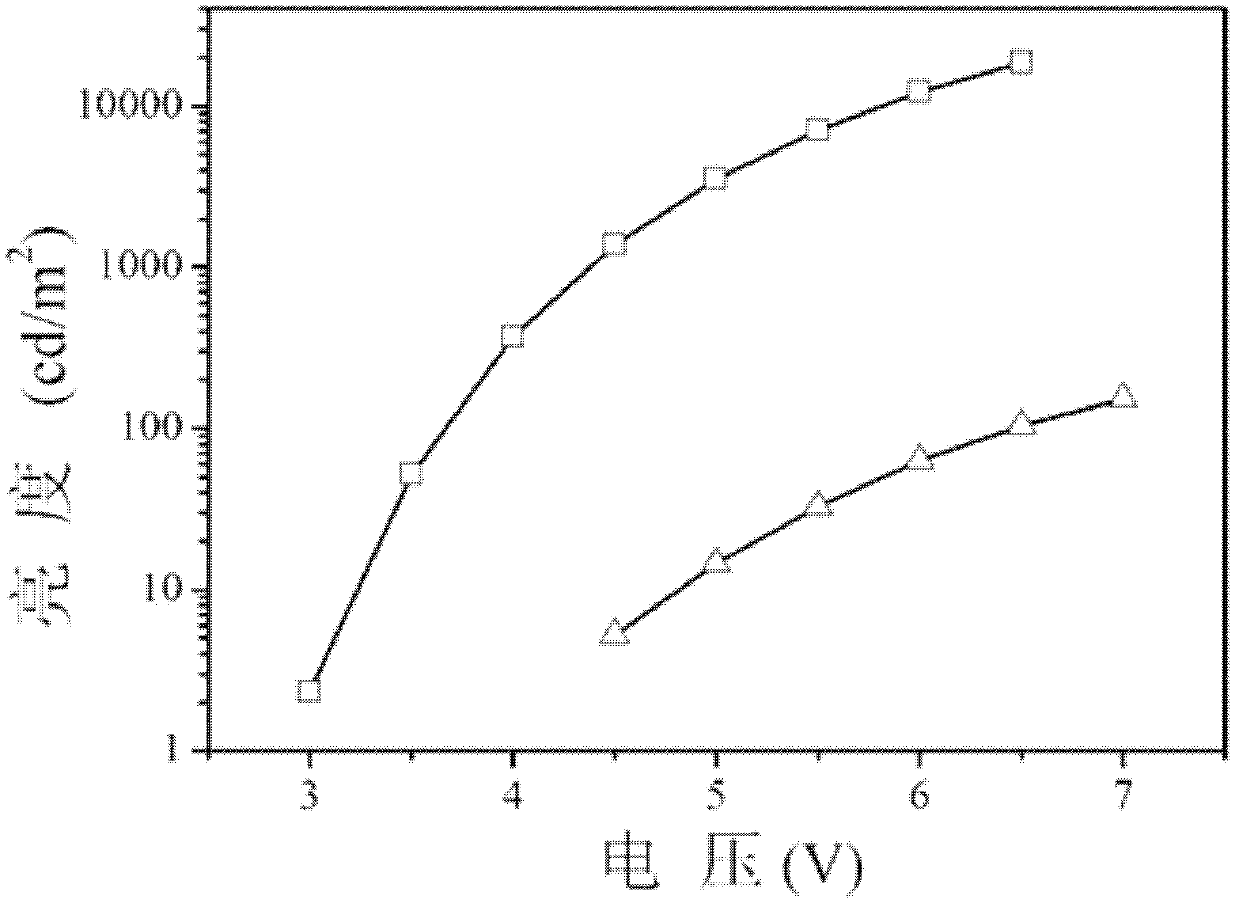
[0037] In this embodiment, dibenzothiophene with a higher triplet energy level is used as the chromophore parent part in the aromatic phosphine oxide structure. Secondly, in this embodiment, the diphenylphosphine group is modified on the chromophore matrix through a C-P ...
specific Embodiment approach 2
[0038] Embodiment 2: This embodiment is different from Embodiment 1 in that X is H. Other parameters are the same as in the first embodiment.
[0039] The dibenzothienyl aromatic phosphine oxide compound of this embodiment is obtained by using dibenzothiophene as a parent, and the diphenylphosphine group is substituted at the 4-position of dibenzothiophene, and its structural formula is as follows:
[0040] Abbreviated as o-DBTSPO.
specific Embodiment approach 3
[0041] Embodiment 3: This embodiment is different from Embodiment 1 in that X is a diphenylphosphine group. Other parameters are the same as in the first embodiment.
[0042] The dibenzothienyl aromatic phosphine oxide compound of this embodiment is obtained by using dibenzothiophene as a parent, and the diphenylphosphine group is substituted at the 4 and 6 positions of dibenzothiophene, and its structural formula is as follows:
[0043] Abbreviated as o-DBTDPO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com