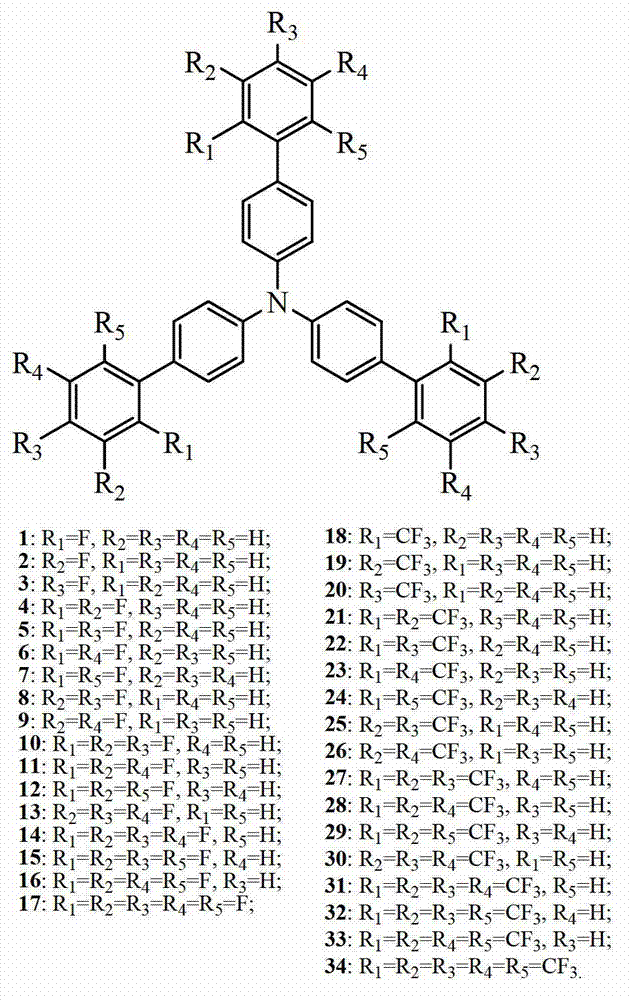
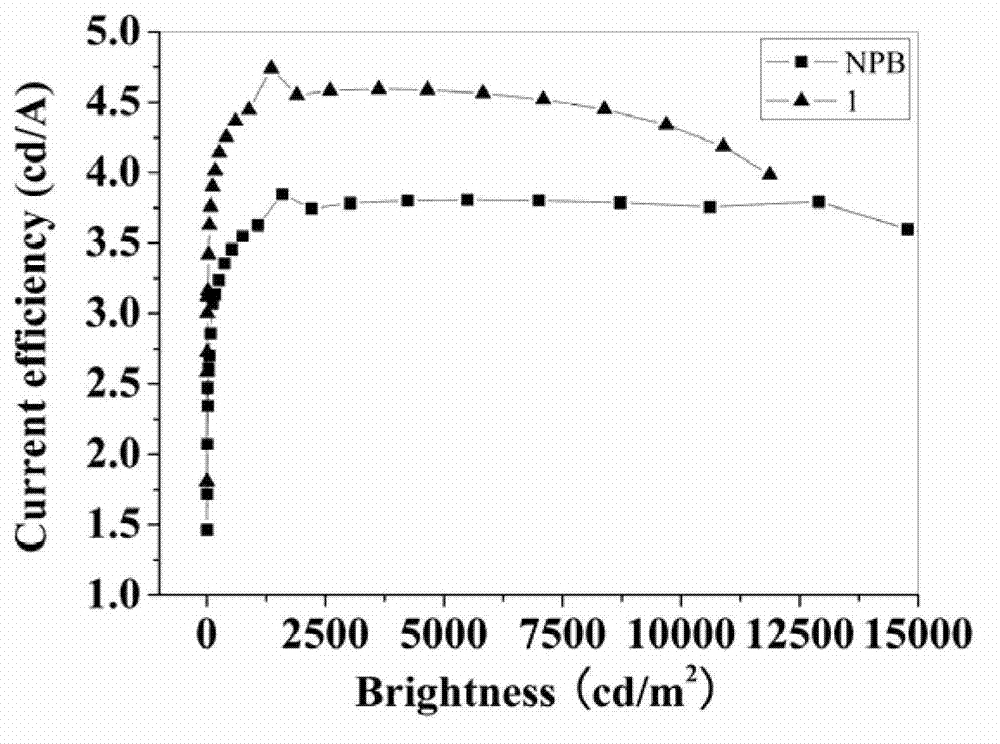
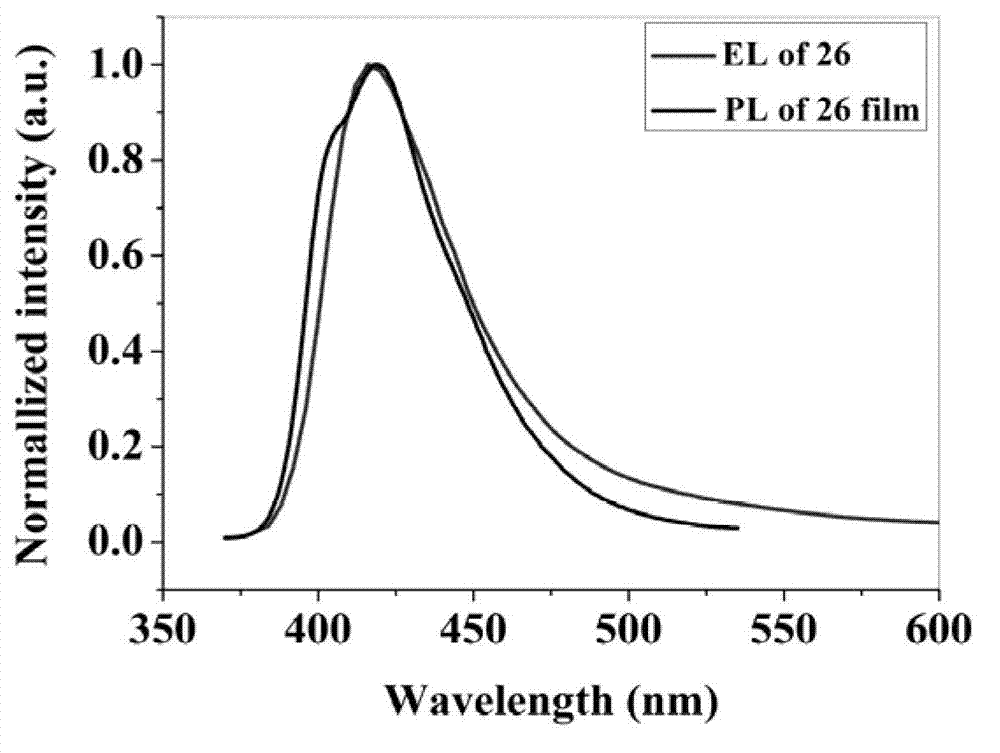
Fluorinated triphenylamine electro-material and application thereof
A technology of triphenylamines and electroluminescent materials, applied in luminescent materials, circuits, photovoltaic power generation, etc., can solve the problem of few blue-violet light-emitting devices, achieve large changes in glass transition temperature range, low turn-on voltage, and simplify devices structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Synthesis of fluorinated triphenylamine material 1:
[0029]
[0030] Under a nitrogen atmosphere, into a 100 mL round bottom flask, mix Na 2 CO 3(2.0M, 15mL), ethanol (10mL) and toluene (30mL), replace nitrogen three times; then add 3mmol tris(4-bromophenyl)amine, 10mmol 2-fluorophenylboronic acid and 0.30mmol tetrakis(triphenylphosphine) ) palladium, replaced nitrogen three times, stirred, heated to reflux, and reacted for 12 hours. The reaction was followed by thin layer chromatography. When the reaction was complete, it was cooled, and 20 mL of water was added to the reaction mixture, extracted three times with dichloromethane, the organic layer was washed with water, and dried over anhydrous sodium sulfate. The organic phase was concentrated, and the pure product 1 was obtained by column chromatography with ethyl acetate and petroleum ether with a yield of 88% (1.39 g). MP: 241°C. 1 H NMR (CDCl 3 , 400MHz): δ7.08-7.17 (m, 3H), 7.24-7.26 (t, 3...
Embodiment 2
[0031] Embodiment 2: Synthesis of fluorinated triphenylamine material 5:
[0032] Under a nitrogen atmosphere, into a 100 mL round bottom flask, mix Na 2 CO 3 (2.0M, 15mL), ethanol (10mL) and toluene (30mL), replace nitrogen three times; then add 5mmol tris(4-bromophenyl)amine, 18mmol 2,4-difluorophenylboronic acid and 0.50mmol tetrakis(triphenyl) Base phosphine) palladium, replace nitrogen three times, stir, heat to reflux, and react for 12 hours. The reaction was followed by thin layer chromatography. When the reaction was complete, it was cooled, and 20 mL of water was added to the reaction mixture, extracted three times with dichloromethane, the organic layer was washed with water, and dried over anhydrous sodium sulfate. The organic phase was concentrated, and the pure product 5 was obtained by column chromatography with ethyl acetate and petroleum ether with a yield of 85% (2.44 g). MP: 162°C. 1 H NMR (CDCl 3 , 400MHz): δ6.88-6.97 (m, 6H), 7.22-7.25 (t, 6H), 7.39-7...
Embodiment 3
[0033] Embodiment 3: Synthesis of fluorinated triphenylamine material 13:
[0034]
[0035] Under a nitrogen atmosphere, into a 100 mL round bottom flask, mix Na 2 CO 3 (2.0M, 15mL), ethanol (10mL) and toluene (30mL), replace nitrogen three times; then add 3mmol tris(4-bromophenyl)amine, 10mmol 3,4,5-fluorophenylboronic acid and 0.30mmol tetrakis( Triphenylphosphine) palladium, replaced nitrogen three times, stirred, heated to reflux, and reacted for 12 hours. The reaction was followed by thin layer chromatography. When the reaction was complete, it was cooled, and 20 mL of water was added to the reaction mixture, extracted three times with dichloromethane, the organic layer was washed with water, and dried over anhydrous sodium sulfate. The organic phase was concentrated, and the pure product 13 was obtained by column chromatography with ethyl acetate and petroleum ether in a yield of 82% (1.56 g). MP: 179°C. 1 H NMR (CDCl 3 , 400MHz): δ7.02-7.04 (d, J=8.8Hz, 3H), 7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com