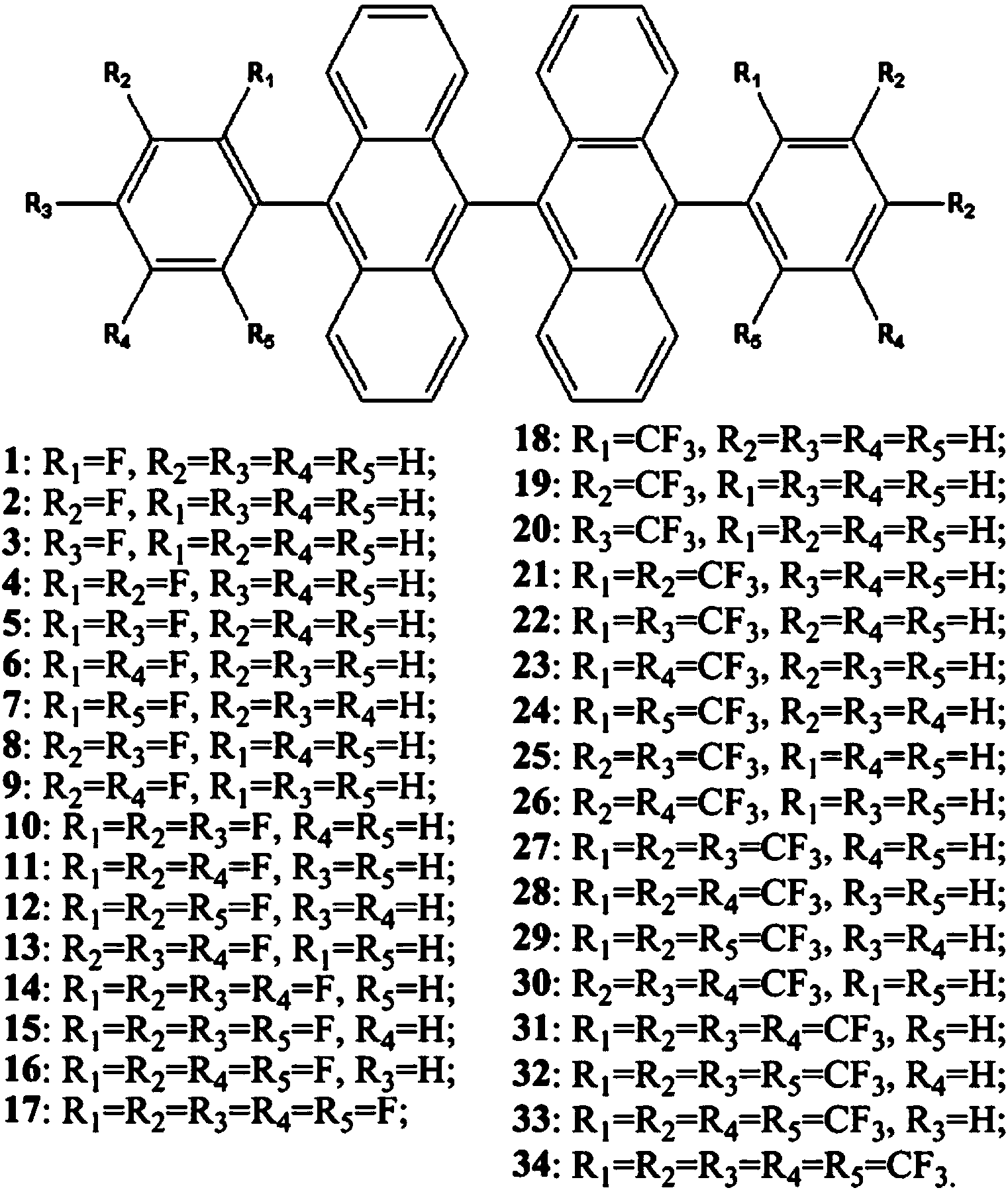
Fluorine-substituted 9,9'-dianthracene blue light emitting host material and its application
A blue-light host material, bianthracene technology, applied in the field of blue-light host materials, fluorine-substituted 9,9'-bianthracene blue-light host materials, can solve the difficulty of phosphorescent blue light devices, and it is difficult to achieve high efficiency, good color purity and long-life blue light Devices and blue light devices have poor performance, and achieve the effects of good thermal stability, high fluorescence quantum efficiency, and low turn-on voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
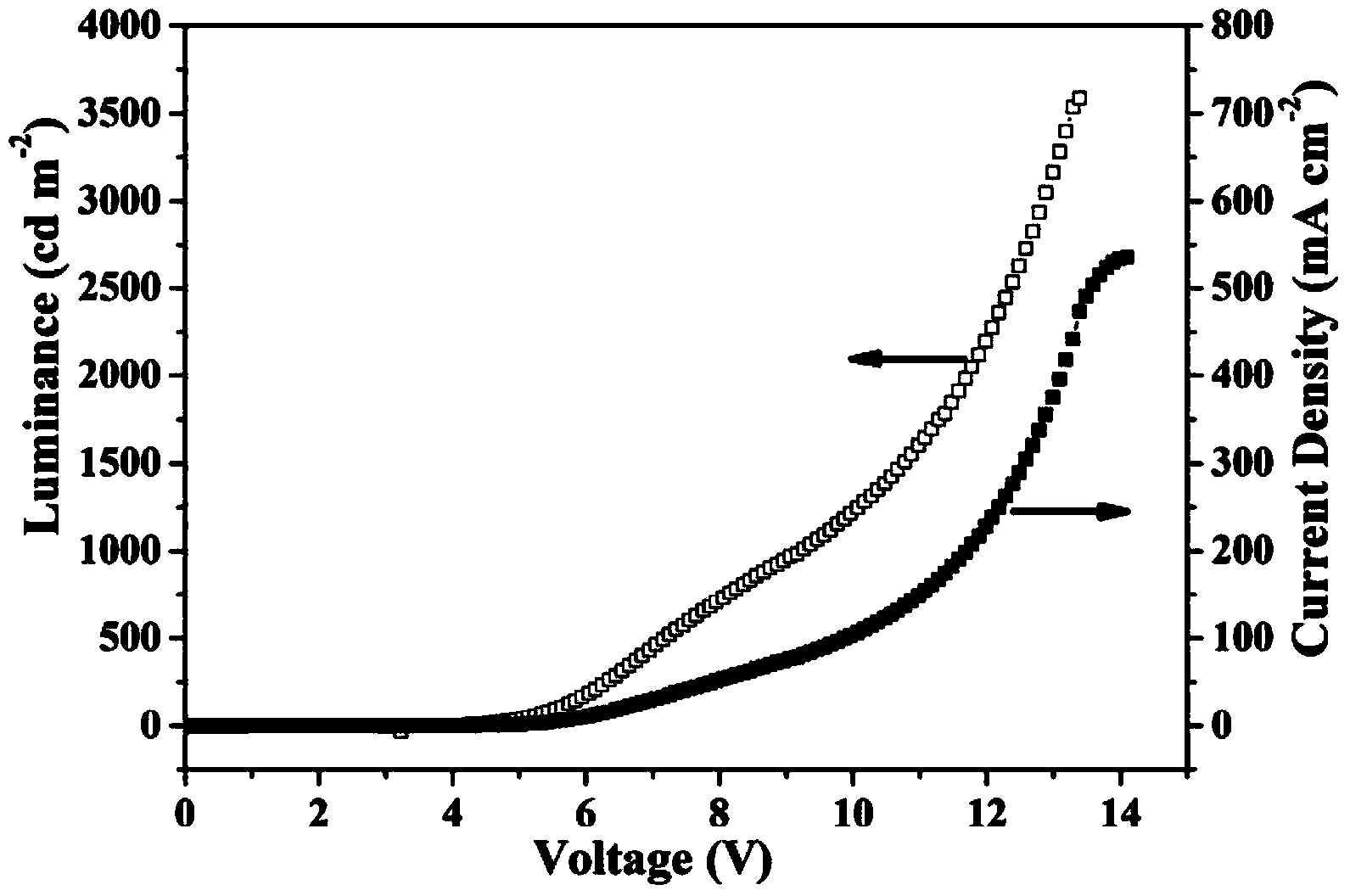
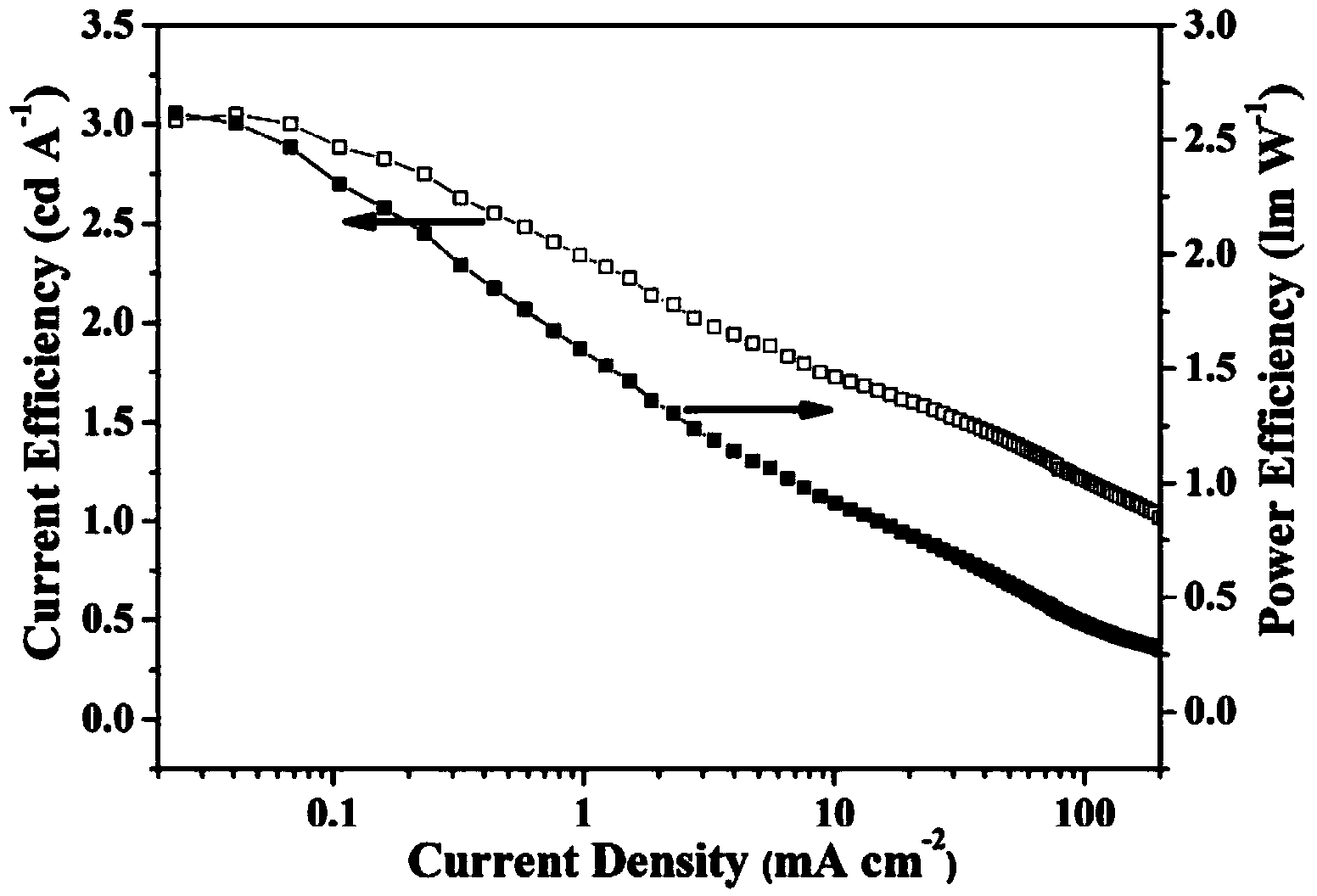
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of fluorinated 9,9'-bianthracene blue light material 1:
[0032]
[0033] Under nitrogen atmosphere, to 30mL THF and 10mL, 2.0mol·L –1 K 2 CO 3 In the solution, 10,10′-dibromo-9,9′-bianthracene (2.34mmol), 2-fluorophenylboronic acid (8mmol) and Pd(PPh 3 ) 4 (0.35 mmol). After the reaction, the mixture was heated to reflux for 24h, and the reaction was tracked by point plate. After the reaction is complete, cool down and add water to stop the reaction. Products with CH 2 Cl 2 Extraction, washing the organic phase with brine, anhydrous MgSO 4 It was dried, concentrated by rotary evaporation to remove the solvent, and 1.13 g of light yellow solid 1 was obtained by column chromatography with ethyl acetate and petroleum ether. 1 H NMR (CDCl 3 ,400MHz):δ7.18-7.26(m,10H),7.27-7.30(m,4H),7.37-7.51(m,4H),7.60-7.68(m,4H),7.78-7.82(d,J= 8.8Hz, 4H).
Embodiment 2
[0034] Example 2: Synthesis of fluorinated 9,9'-bianthracene blue light material 5:
[0035]
[0036] Under nitrogen atmosphere, to 30mL THF and 10mL, 2.0mol·L –1 K 2 CO 3 In the solution, 10,10′-dibromo-9,9′-bianthracene (2.34mmol), 2,4-difluorophenylboronic acid (8mmol) and Pd(PPh 3 ) 4 (0.35 mmol). After the reaction, the mixture was heated to reflux for 24h, and the reaction was tracked by point plate. After the reaction is complete, cool down and add water to stop the reaction. Products with CH 2 Cl 2 Extraction, washing the organic phase with brine, anhydrous MgSO 4 It was dried, concentrated by rotary evaporation to remove the solvent, and 1.22 g of light yellow solid 5 was obtained by column chromatography with ethyl acetate and petroleum ether. 1 H NMR (CDCl 3 ,400MHz): δ7.17-7.23(m,12H),7.38-7.41(m,4H),7.53-7.61(q,4H),7.74-7.61(d,J=8.0Hz,4H).
Embodiment 3
[0037] Example 3: Synthesis of fluorinated 9,9'-bianthracene blue light material 13:
[0038]
[0039] Under nitrogen atmosphere, to 30mL THF and 10mL, 2.0mol·L –1 K 2 CO 3 In the solution, 10,10′-dibromo-9,9′-bianthracene (2.34mmol), 2,3,4tri-fluorophenylboronic acid (8mmol) and Pd(PPh 3 ) 4 (0.35 mmol). After the reaction, the mixture was heated to reflux for 24h, and the reaction was tracked by point plate. After the reaction is complete, cool down and add water to stop the reaction. Products with CH 2 Cl 2 Extraction, washing the organic phase with brine, anhydrous MgSO 4 It was dried, concentrated by rotary evaporation to remove the solvent, and 1.38 g of white solid 13 was obtained by column chromatography with ethyl acetate and petroleum ether. 1 H NMR (CDCl 3 ,400MHz): δ7.18-7.20(m,8H),7.27-7.31(t,4H),7.38-7.43(m,4H),7.73-7.78(d,J=8.8Hz,4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com