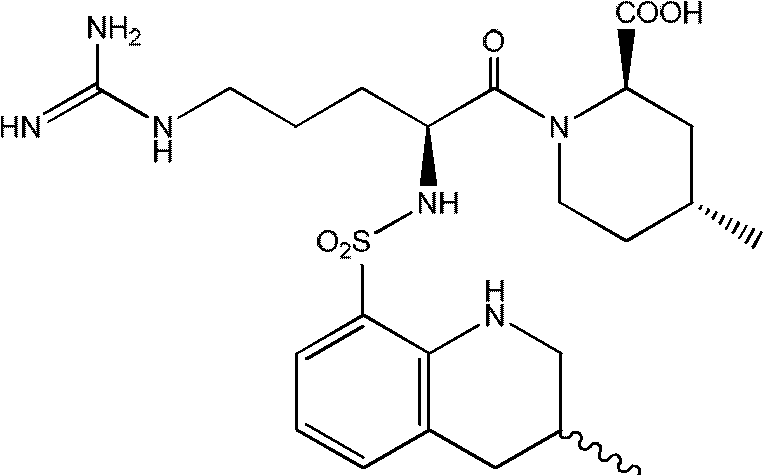
Method for separating single stereoisomer of Argatroban and polymorphic substance
An argatroban, separation technology, applied in the preparation of polymorph A and polymorph B, the application field of preparation of anticoagulant and antithrombotic drugs, can solve the problem of increased side effects and anticoagulant effect Advanced problems, to achieve the effect of saving energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
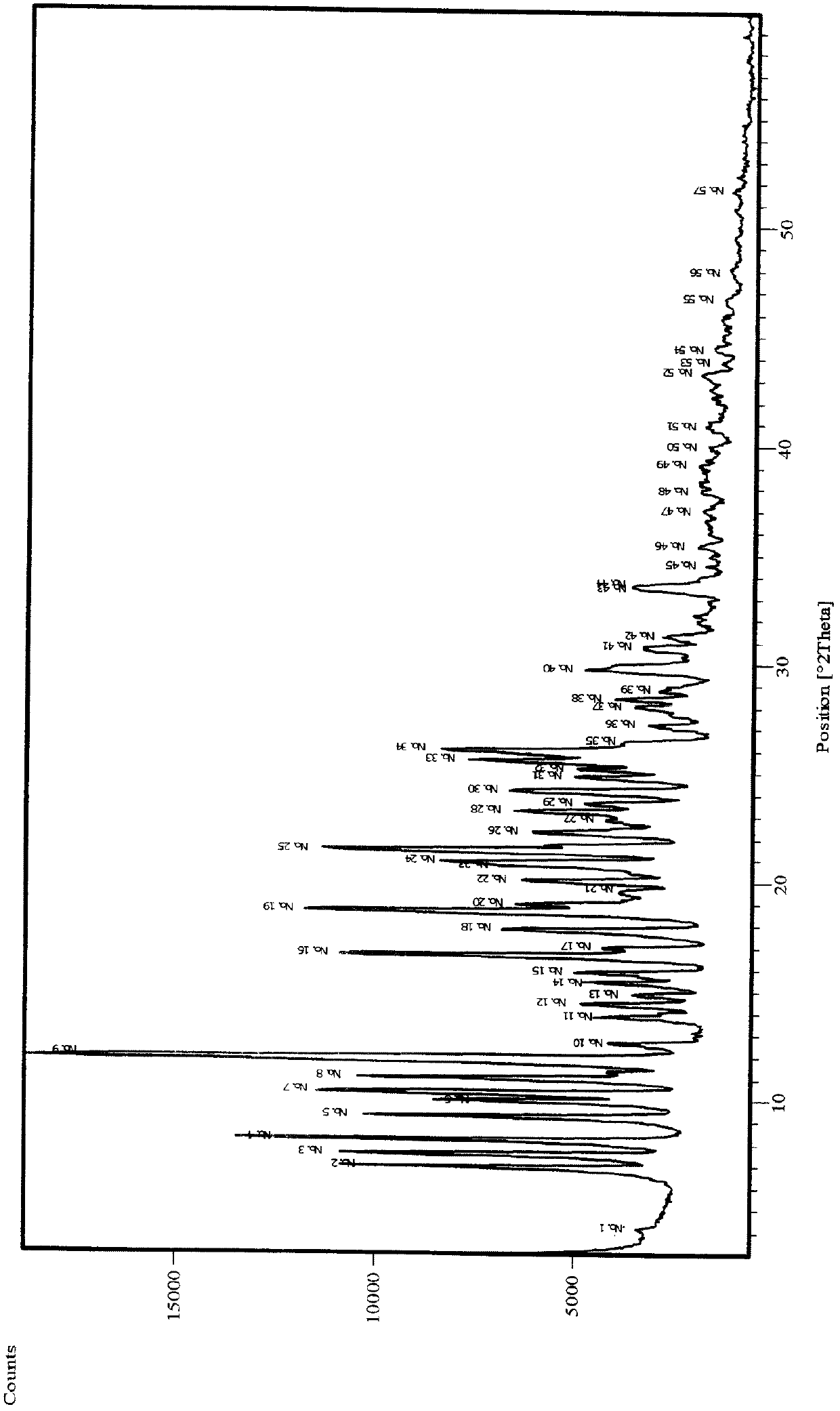
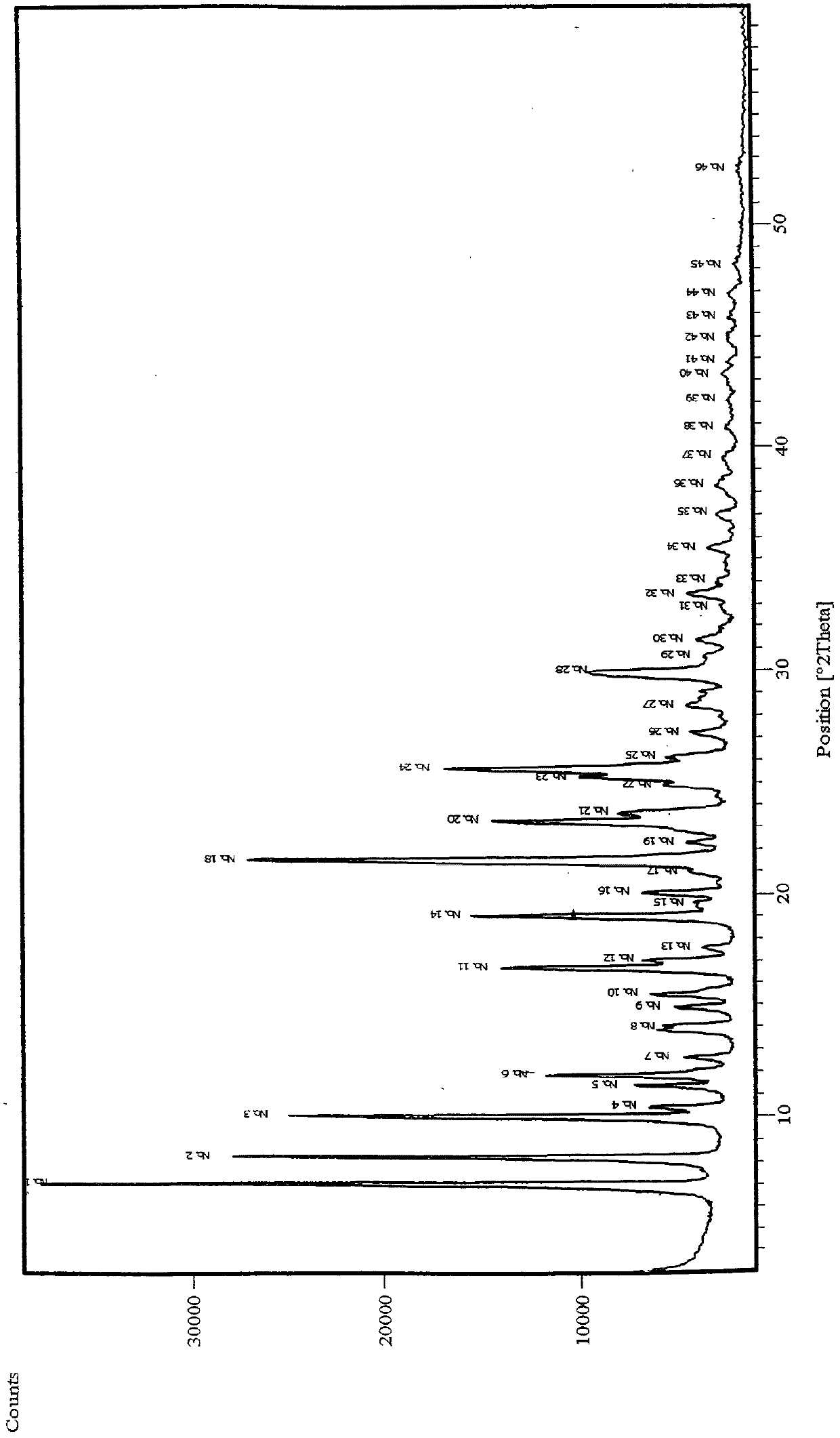
Image
Examples
Embodiment 1
[0042] Dissolve 10g argatroban (compound II content 35%) in 80ml methanol at 60°C, add 16ml pure water to the solution, mix well and slowly cool to room temperature, leave it overnight and then precipitate crystals, filter and collect It was dried to constant weight under vacuum to obtain 7.5 g of solid. HPLC analysis showed that the content of compound II was 45%.
Embodiment 2
[0044] Dissolve 1g argatroban (compound II content 35%) in 10ml methanol at 60°C, add 2ml absolute ethanol to the solution, mix well and slowly cool to room temperature, leave it for 1 hour and then precipitate crystals, filter After collection, it was dried to constant weight under vacuum to obtain 0.7 g of solid. HPLC analysis showed that the content of compound II was 41%.
Embodiment 3
[0046] Dissolve 1g argatroban (compound II content 35%) in 10ml methanol at 60°C, add 2ml anhydrous ether to the solution, mix well and slowly cool to room temperature, leave it for 1 hour and then precipitate crystals, filter After collection, it was dried to constant weight under vacuum to obtain 0.78 g of solid. HPLC analysis showed that the content of compound II was 39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com