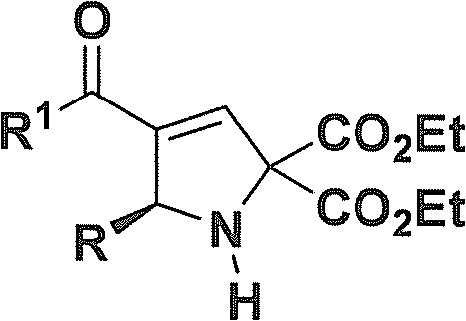
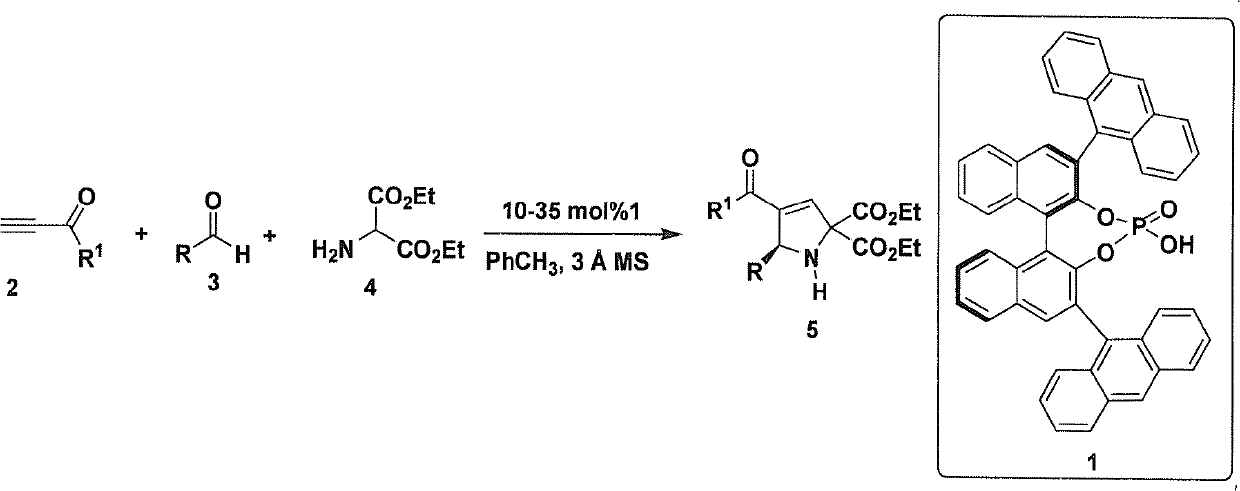
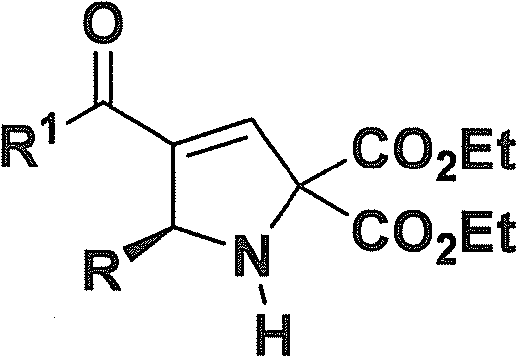
Chiral 2,5-pyrroline derivative, and synthetic method and biological activity thereof
A technology of dihydropyrrole and derivatives, applied in the field of chirality, which can solve the problems of uneconomical and easy availability of chiral raw materials, cumbersome operation, and limited methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The (R)-type chiral phosphoric acid catalyst represented by 0.12mmol of 4-nitrobenzaldehyde, 0.1mmol of 2-aminomalonate, 0.01mmol of formula 1, 100mg Molecular sieves (purchased from Tianjin Alpha Aisha Co., Ltd.) were put into a 10 mL glass reaction test tube, 1 mL of toluene was added, stirred at room temperature for 10 minutes (600 rpm / min), then cooled to -10 °C, and then 0.5 mmol of 3-butylene was added Alkyn-2-one, react at -10°C for 30 hours.
[0057] Then, 4 mL of ethyl acetate was added to the test tube containing the reaction mixture to dilute the reaction mixture. Spread a layer of thin-layer chromatography silica gel H on a glass funnel plugged with cotton, pump the silica gel tightly with a water pump, then pour the reaction mixture in the test tube into the funnel, and filter out molecular sieves, including molecular sieves. A layer of chromatographic silica gel remains in the funnel as a filter cake. Next, after washing the suction-filtered filter cake...
Embodiment 2
[0062] Adopt the method identical with embodiment 1, wherein: the aldehyde that adopts is 3-nitrobenzaldehyde, add thin-layer chromatography silica gel H in common glass column, pressurize column chromatography with nitrogen (column length 15 centimetres, flow velocity 3 drop / second), the eluent was petroleum ether: ethyl acetate 6:1 (volume ratio), and the product 5ab was obtained with a yield of 88% and an ee of 92%.
[0063] Characterization data of compound 5ab:
[0064]
[0065] (R)-Diethyl 4-acetyl-5-(3-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylate: colorless oil; [α] D 20 =+249.2(c 0.3, CHCl 3 ); 1 H-NMR (CDCl 3, 400MHz) δ(ppm): 8.21(t, J=8.0Hz, 1H, ArH), 8.10-8.07(m, 1H, ArH), 7.69(d, J=7.6Hz, 1H, ArH), 7.45(t , J=8.0Hz, 1H, ArH), 6.82(d, J=2.0Hz, 1H, =CH), 5.52(d, J=1.6Hz, 1H, CH), 4.38-4.26(m, 4H, 2CH 2 ), 3.64(s, 1H, NH), 2.32(s, 3H, CH 3 ), 1.34 (q, J = 7.2Hz, 6H, 2CH 3 ); 13 C-NMR (CDCl 3 , 100MHz) δ (ppm): 194.0, 169.1, 169.0, 148.4, 147.2, 144.4, 13...
Embodiment 3
[0067] Adopt the method identical with embodiment 1, wherein: the aldehyde that adopts is 2-nitrobenzaldehyde, adds thin-layer chromatography silica gel H in common glass column, pressurized column chromatography with nitrogen (column length 15 centimetres, flow velocity 3 drop / second), the eluent was petroleum ether: ethyl acetate 6:1 (volume ratio), and the product 5ac was obtained with a yield of 87% and an ee of 90%.
[0068] Characterization data of compound 5ac:
[0069]
[0070] (R)-Diethyl 4-acetyl-5-(2-nitrophenyl)-1H-pyrrole-2,2(5H)-dicarboxylate: yellow oil; [α] D 20 =+364.4 (c 0.4, CHCl 3 ); 1 H-NMR (CDCl 3 , 400MHz) δ (ppm): 7.83 (dd, J 1 =8.0Hz,J 2 =1.2Hz, 1H, ArH), 7.50(dt, J 1 =8.2Hz,J 2 =1.2Hz, 1H, ArH), 7.39-7.33(m, 2H, ArH), 6.85(d, J=2.0Hz, 1H, =CH), 6.08(dd, J 1 =6.4Hz,J 2 =2.0Hz, 1H, CH), 4.38-4.20 (m, 4H, 2CH 2 ), 3.91(d, J=6.4Hz, 1H, NH), 2.31(s, 3H, CH 3 ), 1.33(t, J=7.2Hz, 3H, CH 3 ), 1.29(t, J=7.2Hz, 3H, CH 3 ); 13 C-NMR (CDCl 3 , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com