Method for purifying recombinant proteins with intein-mediated elastin like proteins
An elastin-like and recombinant protein technology, applied in the field of recombinant drug protein purification, can solve problems such as difficulty, cost increase, limited clinical application, etc., and achieve the effect of simple operation and high trial cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
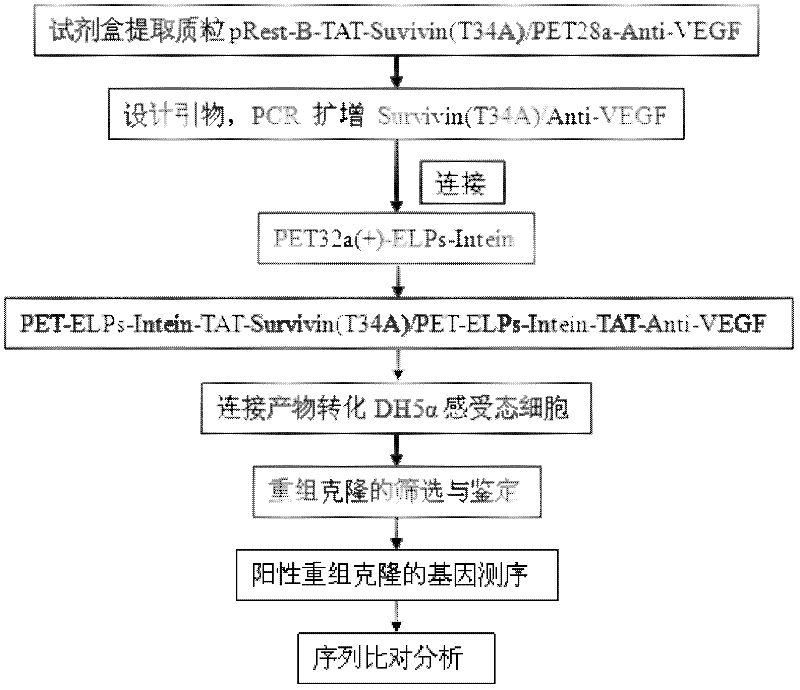
[0030] Example 1: Intein-mediated elastin-like purification system Purification of recombinant protein TAT-Survivin (T34A) and TAT-Anti-VEGF single-chain antibody (ScFv).
[0031] 1. PCR amplification of the TAT-Survivin(T34A) / TAT-Anti-VEGF single-chain antibody gene.
[0032] According to the sequences of plasmids pRest-TAT-Survivin (T34A), PET28a-Anti-VEGF single-chain antibody and PET-ELP-intein (given to Dr. Wood from Duke University, USA) constructed in our laboratory, the following primers were designed For the amplification of the above genes. Its sequence is shown in Table 1 below. The primers used were synthesized by BGI Corporation.
[0033] Table 1 Primers used in this experiment
[0034]
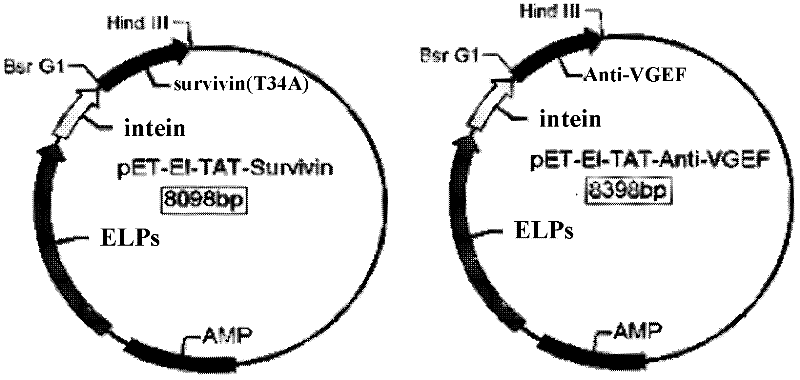
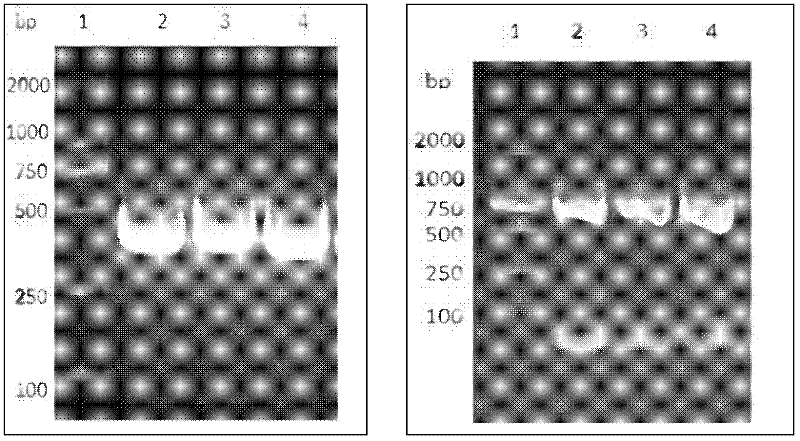
[0035] The experiment technique of molecular biology was used, and the specific method was carried out in "Molecular Cloning". Build according to the following technical routes and methods. figure 1 Construct a schematic road map for the plasmid, and finally construct a g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com