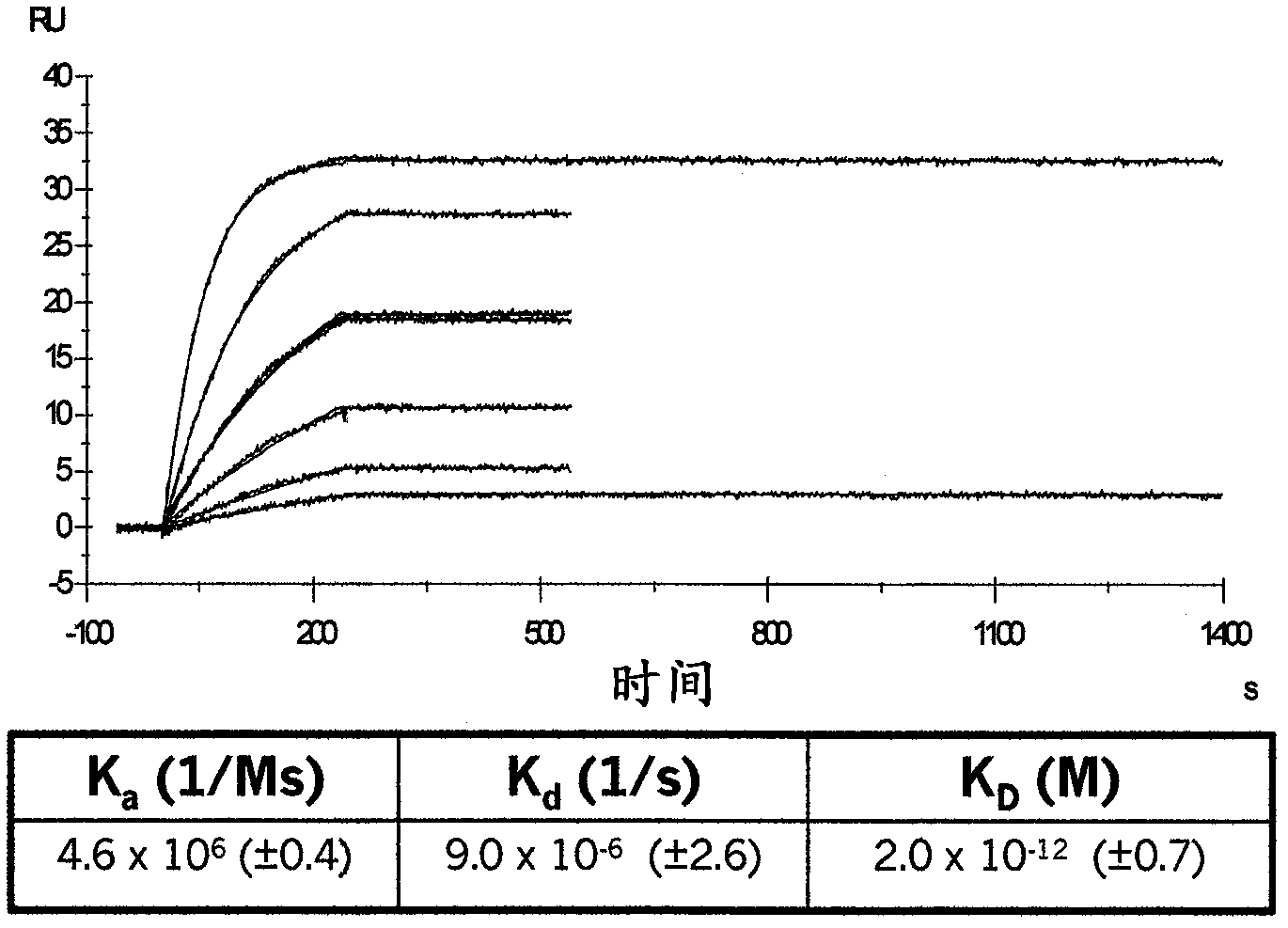
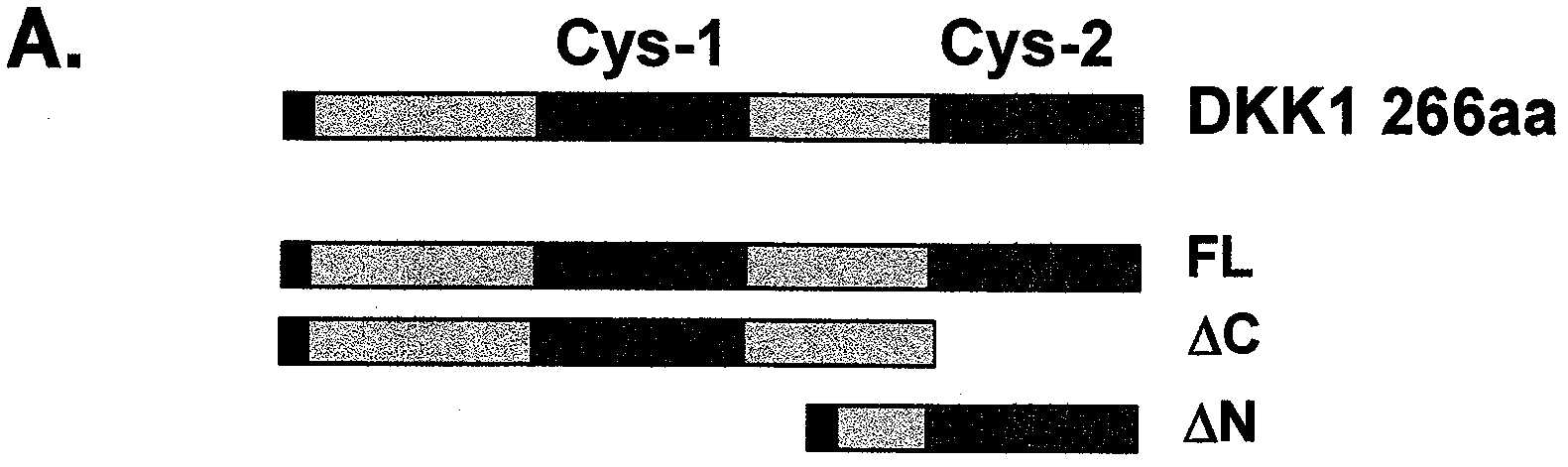
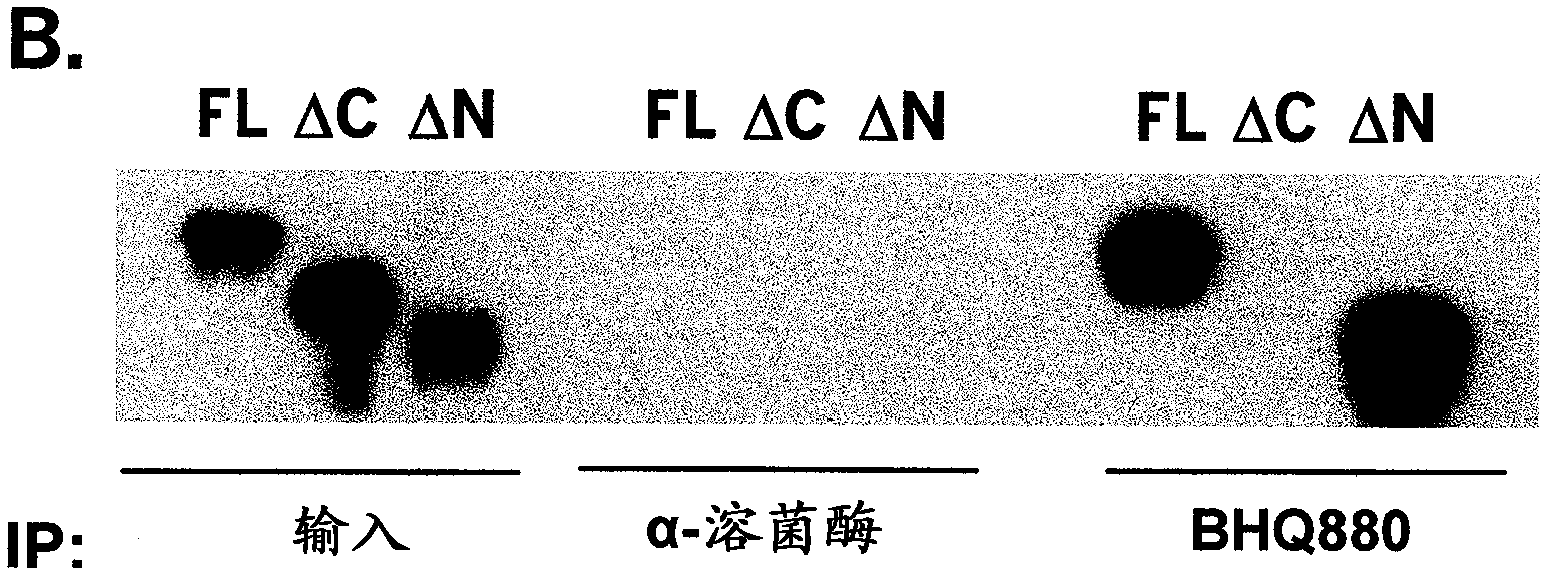
Compositions and methods of use for binding molecules to dickkopf-1 or dickkopf-4 or both
A technology of DKK1, a composition, applied in the field of expression-related disorders or conditions, capable of solving problems such as poor characterization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0313] Example 1: From HuCAL GOLD Preparation of human DKK1-specific antibody in the library
[0314] Therapeutic antibodies against human DKK1 protein can be obtained by using the commercially available phage display library MorphoSys HuCAL GOLD as a source of antibody variant protein library to select clones with high binding affinity for generation. HuCAL GOLD is a Fab library (Knappik et al., 2000 J. Mol. Biol. 296:57-86; Krebs et al., 2001 J Immunol. Methods 254:67-84; Rauchenberger et al., 2003 J Biol Chem.278(40):38194 -38205), in which all six CDRs were diversified by appropriate mutations, and CysDisplay TM Techniques link Fab fragments to the surface of phage (WO 01 / 05950 Lδhning 2001).
[0315] Routine procedures: phagemid recovery, phage amplification, and purification
[0316] HuCAL GOLD Libraries were amplified in standard rich bacterial medium (2xYT) containing 34 μg / ml chloramphenicol and 1% glucose (2xYT-CG). in OD 600nm When the temperature was 0....
Embodiment 2
[0345] Example 2: DKK1 specific HuCAL Antibody identification
[0346] The BEL extracts of each Escherichia coli clone selected by the above panning strategy were analyzed by microvolume fluorescence assay technique (FMAT TM , 8200 Cellular Detection System analyzer, Applied Biosystems, Foster City, Calif) analysis to identify clones encoding DKK1-specific Fab. FM AT TM The 8100 HTS system is a confocal high-throughput fluorescence screening instrument that automatically detects the mixing and reading of living cells or beads, and is a non-radioactive assay (Miraglia, J. Biomol. Screening (1999), 4(4) 193-204 ).
[0347] A microvolume-based fluorescence assay technique for detecting DKK1-bound Fabs from bacterial lysates (FMAT) binding assay
[0348] For detection of DKK1-bound Fab antibodies from E. coli lysates (BEL extracts), binding was analyzed using a FMAT 8200 cell detection system (Applied Biosystems). To bind His-Strep-tagged DKK1 to M-450Expoxy beads (Dyna...
Embodiment 3
[0358] Example 3: Identification of anti-human DKK1 Fab candidates that inhibit the Wnt antagonistic activity of DKK1
[0359] Use obtained from HuCAL GOLD A library of 57 different DKK1-specific antibodies was obtained to obtain purified antibodies, which were then used to test the ability to inhibit the Wnt antagonistic activity of human DKK1. Seventeen of these antibody candidates were functionally active.
[0360] Detection of each HuCAL using a luciferase reporter assay Functional activity of the Fab. Twelve binding sites for TCF / Lef were cloned upstream of a luciferase reporter gene that exhibited a luciferase gene TCF / Lef response. Regular canonical Wnt proteins lead to the stabilization of β-catenin, which activates the transcription of TCF / Lef and produces the luciferase protein. Addition of the DKK1 protein blocks Wnt activity and thus also transcription of the luciferase gene. As a result, the level of luciferase produced by individual cells is expected to co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com