Methods of treatment using combination therapy
A compound and leukemia technology, applied in the direction of drug combination, pharmaceutical formulation, active ingredient of heterocyclic compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0947] Example 1. Clinical research:
[0948] A clinical trial was carried out to determine azacytidine (Aza) and N-(5-tert-butyl-isoxazol-3-yl)-N'-{4-[7-(2-morpholin-4-yl- Clinical activity and efficacy of ethoxy)imidazol[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea (AC220) in the treatment of refractory or relapsed AML and / or MDS patients Toxic features. In addition to clinical feedback, patients can be tested for specific related studies, such as induction of hypomethylation, DNA damage, and FLT-3 signaling.
[0949] The trial was a phase I / II, single-arm, open-label study in which patients received treatment in each 28-day treatment cycle consisting of daily oral administration of the compound of formula (I) or AC220 for the first 14 days , Aza was given subcutaneously or intravenously daily for the first 7 days. Courses were cycled approximately every 28 days, and treatment was continued until disease progression or unacceptable toxicity was documented.
[0950] Approxima...
Embodiment 2
[0975] The effectiveness experiment of AC220 plus arabinocytosine glycoside in MV4-11 solid tumor flank model
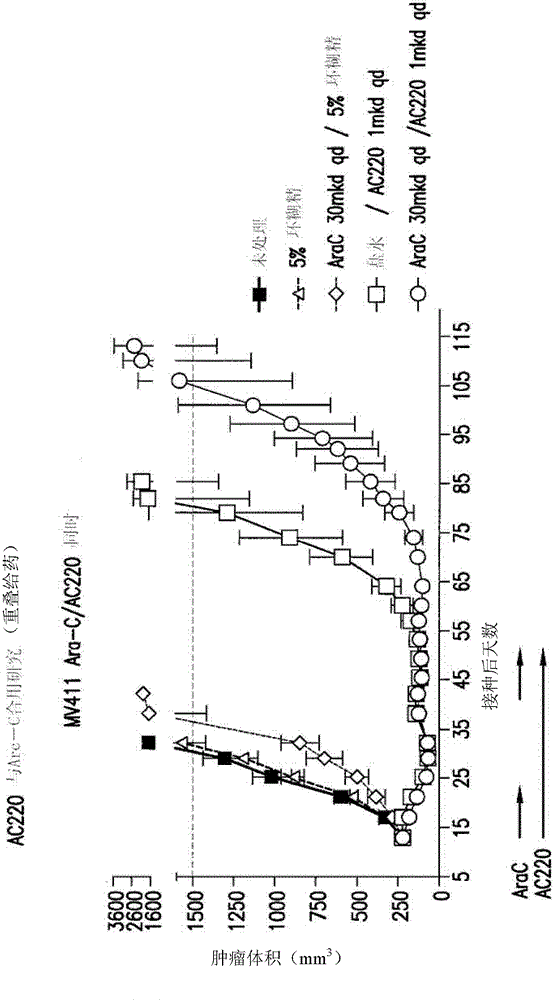
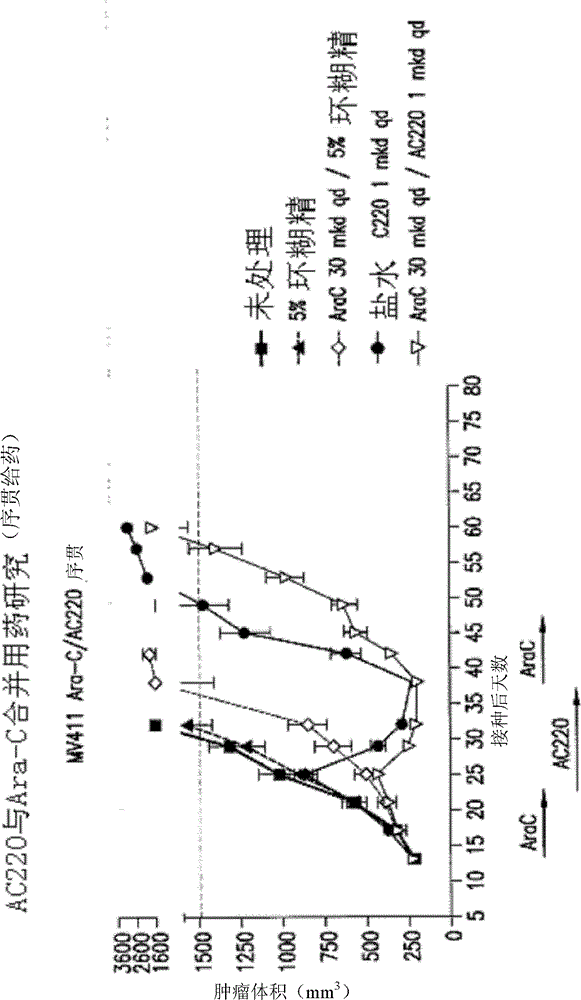
[0976] Efficacy experiments (in vivo) of AC220 plus cytosine arabinoside were performed in the MV4-11 solid tumor flank model using SCID mice. Dosing to groups of 10 animals per arm began 14 days after inoculation. The average size of the tumor is about 222mm 3 . AC220 was delivered at 1.0 mg / kg / day (mkd) QD, PO, and body weight-adjusted doses in 5% hydroxy-β-cyclodextrin in water (5 ml / kg / day, prepared weekly). Cytosine arabinoside was administered in sterile saline (5 ml / kg / day, prepared weekly) at about 30 or about 60 mkd, QD, IP, and body weight adjusted doses. The average starting body weight for each group was about 20 g. Clinical signs and body weights were measured twice weekly. White blood cell (WBC) counts were measured at the end of the cytosine arabinoside administration period and again after 7 days of drug treatment. The study was conducted in one...
Embodiment 3
[0982] In vivo studies of AC220 plus Aza-C in the MV4-11 solid tumor flank model
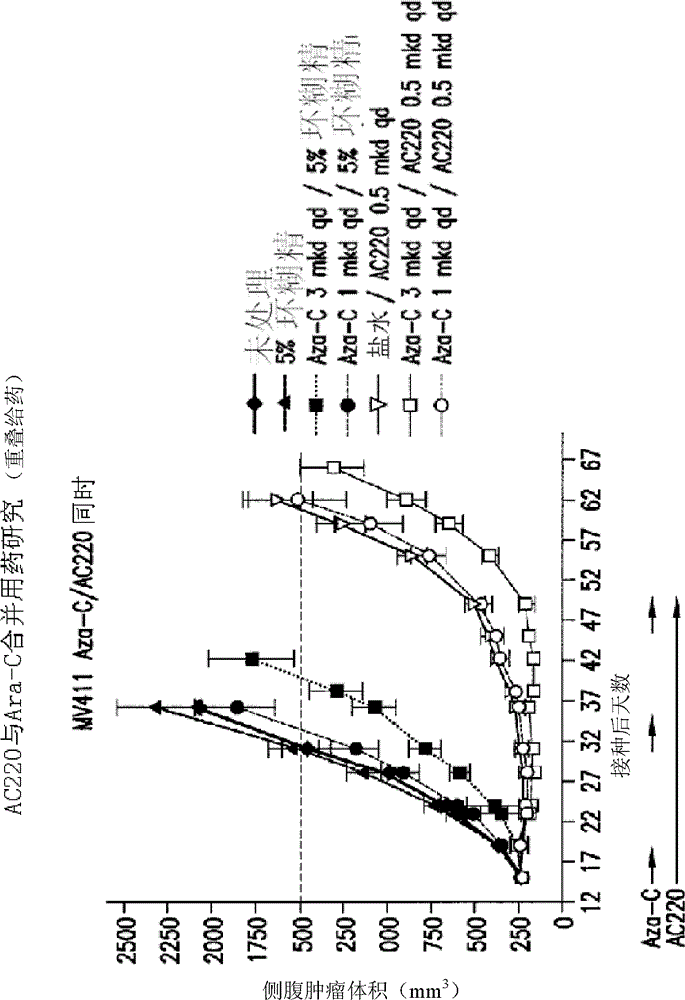
[0983]The efficacy study (in vivo) of AC220 plus Aza-C was performed in the MV4-11 solid tumor flank model in SCID mice. Dosing began 15 days after inoculation to groups of 10 animals per arm. The average size of the tumor is about 230mm 3 . AC220 is delivered at 0.5 mg / kg / day (mkd) QD, PO, and body weight-adjusted doses in 5% hydroxy-β-cyclodextrin in water (10 ml / kg / day, prepared weekly). Aza-C was administered in sterile saline (10 ml / kg / day, prepared in 5-day batches) at about 3 or about 1 mkd, QD, IP, and body weight adjusted doses. The average starting body weight for each group was approximately 20.6 g. Clinical signs and body weights were measured twice weekly. White blood cell (WBC) counts were measured at the end of the cytosine arabinoside administration period and again after 7 days of drug treatment. The study was conducted in one or more treatment cycles, with Aza-C administe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com