Alkane hydroisomerization catalyst and preparation method and application thereof
A kind of hydroisomerization and catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalyst, molecular sieve catalyst, etc., can solve the problems of high cracking rate, insufficient activity, shallow degree of isomerization, etc., and achieve high conversion activity, The effect of low cleavage activity selectivity and deep isomerism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Tetraethylammonium hydroxide (TEAOH) is used as template, and the raw materials are borax, sodium hydroxide and silica gel.
[0057] The molar ratio of feeding is:
[0058] SiO 2 / B 2 o 3 =30;TEAOH / B 2 o 3 =3.6; Al 2 o 3 / B 2 o 3 =0
[0059] Na 2 O / B 2 o 3 = 2; H 2 O / B 2 o 3 =200.
[0060] The first stage reaction conditions: 120°C, time: 20 hours
[0061] Second stage reaction conditions: 140°C, time: 40 hours
[0062] The third stage reaction condition: 180°C, time: 8 hours.
[0063] The synthetic sample is B-beta sample A.
Embodiment 2
[0065] Tetraethylammonium hydroxide (TEAOH) is used as a template, and the raw materials are boric acid, sodium hydroxide and water glass.
[0066] The molar ratio of feeding is:
[0067] SiO 2 / B 2 o 3 =55;TEAOH / B 2 o 3 =1.8; Al 2 o 3 / B 2 o 3 =0.5
[0068] Na 2 O / B 2 o 3 =12;H 2 O / B 2 o 3 =400.
[0069] The first stage reaction conditions: 140°C, time: 30 hours
[0070] Second stage reaction conditions: 150°C, time: 48 hours
[0071] The reaction condition of the third stage: 180°C, the time: 20 hours.
[0072] The main properties of the products obtained are listed in Table 1.
[0073] The B-beta sample B was obtained.
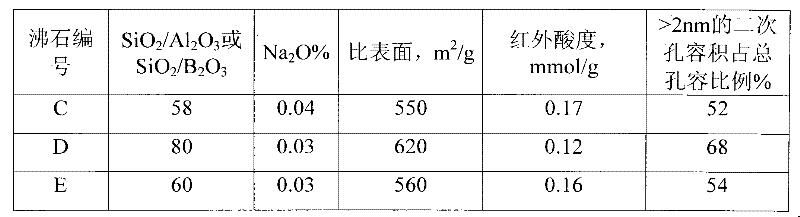
[0074] Table 1 Main physicochemical properties of B-β products
[0075] sample
Embodiment 3
[0077] The invention synthesizes modified B-beta zeolite C. Take 2000ml of A sample slurry, containing 400g of solid phase (on a dry basis), dilute the solid-liquid volume ratio to 1:10 with clean water, then add ammonium nitrate to make the concentration of ammonium nitrate in the solution 2.0M, stir, and heat up to 90 Stir at ~95°C for 2 hours at a constant temperature, then cool down to 50°C for filtration, and perform a second exchange of the wet filter cake under the same conditions as the first. The B-beta zeolite that has been exchanged twice with ammonium salts is washed until the pH reaches 5-6, and then placed in a drying oven and dried at 110-120° C. for 6 hours (dry basis is 88% by weight). The dried B-β zeolite is placed in a muffle furnace and rapidly raised to 250°C, kept at a constant temperature for 2 hours, then rapidly raised to 400°C, kept at a constant temperature for 4 hours, and finally raised to 540°C, kept at a constant temperature for 10 hours. After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com