Positive electrode for secondary batteries, and secondary battery
A secondary battery and positive electrode technology, applied in the direction of secondary batteries, battery electrodes, non-aqueous electrolyte batteries, etc., can solve the problems of battery capacity degradation, battery safety reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
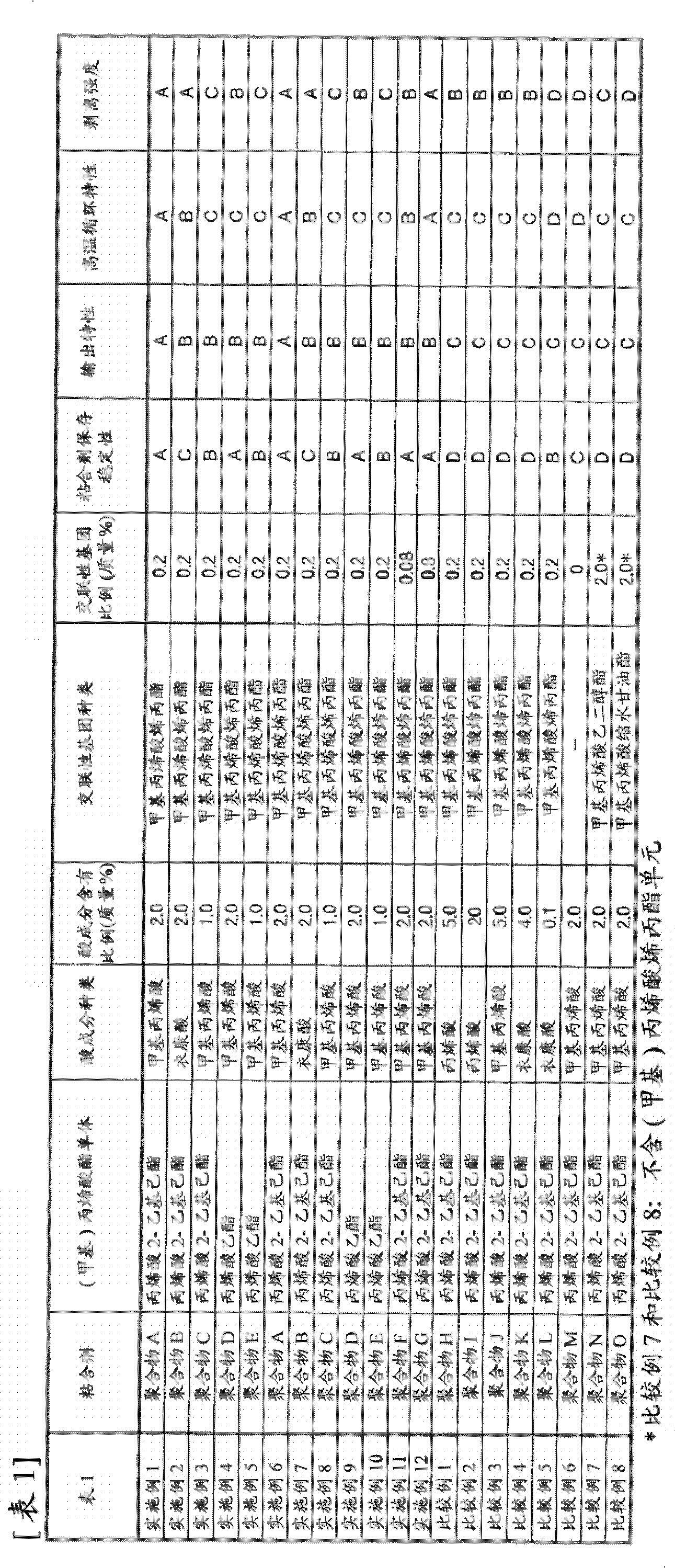
Image
Examples
Embodiment 1
[0149] (A) Manufacture of adhesive
[0150] 10.75 parts of 2-ethylhexyl acrylate, 1.25 parts of acrylonitrile, 0.12 parts of sodium lauryl sulfate, and 79 parts of ion-exchanged water were added to polymerization tank A, and then 0.2 parts of ammonium persulfate and ion-exchanged water were added as polymerization initiators. 10 parts, heated to 60°C and stirred for 90 minutes, then, 67 parts of 2-ethylhexyl acrylate, 19 parts of acrylonitrile, 2.0 parts of methacrylic acid, and allyl methacrylate were added to another polymerization tank B 0.2 part, 0.7 part of sodium lauryl sulfate, and 46 parts of ion-exchanged water were stirred to produce a latex, and the produced latex was gradually added from the polymerization tank B to the polymerization tank A over about 180 minutes, and then stirred for about 120 minutes, When the monomer consumption reached 95%, the reaction was terminated by cooling, and then the pH was adjusted with a 4% NaOH aqueous solution to obtain an aqueous...
Embodiment 2
[0159] (A) Manufacture of adhesive
[0160] 10.75 parts of 2-ethylhexyl acrylate, 1.25 parts of acrylonitrile, 0.12 parts of sodium lauryl sulfate, and 79 parts of ion-exchanged water were added to polymerization tank A, and then 0.2 parts of ammonium persulfate and ion-exchanged water were added as polymerization initiators. 10 parts, heated to 60°C and stirred for 90 minutes, then, 67 parts of 2-ethylhexyl acrylate, 19 parts of acrylonitrile, 2.0 parts of itaconic acid, and allyl methacrylate were added to another polymerization tank B 0.2 part, 0.7 part of sodium lauryl sulfate, and 46 parts of ion-exchanged water were stirred to produce a latex, and the produced latex was gradually added from the polymerization tank B to the polymerization tank A over about 180 minutes, and then stirred for about 120 minutes, When the monomer consumption reached 95%, cooling was performed to terminate the reaction, and then the pH was adjusted with a 4% aqueous NaOH solution to obtain an a...
Embodiment 3
[0164] (A) Manufacture of adhesive
[0165] 10.75 parts of 2-ethylhexyl acrylate, 1.25 parts of acrylonitrile, 0.12 parts of sodium lauryl sulfate, and 79 parts of ion-exchanged water were added to polymerization tank A, and then 0.2 parts of ammonium persulfate and ion-exchanged water were added as polymerization initiators. 10 parts, heated to 60° C. and stirred for 90 minutes, then, 67 parts of 2-ethylhexyl acrylate, 19 parts of acrylonitrile, 1.0 part of methacrylic acid, and allyl methacrylate were added to another polymerization tank B 0.2 part, 0.7 part of sodium lauryl sulfate, and 46 parts of ion-exchanged water were stirred to produce a latex, and the produced latex was gradually added from the polymerization tank B to the polymerization tank A over about 180 minutes, and then stirred for about 120 minutes, When the monomer consumption reached 95%, the reaction was terminated by cooling, and then the pH was adjusted with a 4% NaOH aqueous solution to obtain an aqueou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Dispersed particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com