Amphiphilic anti-cancer drug compound modified by water-soluble vitamin E derivative, preparation, preparation method and application for compound
A technology of water-soluble vitamins and anti-cancer drugs, which is applied to compounds, chemical instruments and methods, and drug combinations of elements in group 5/15 of the periodic table, and can solve problems such as poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
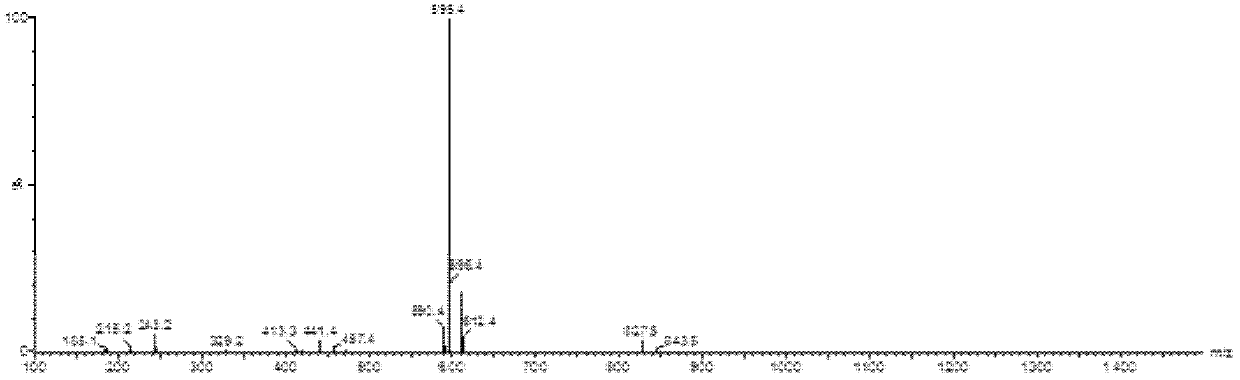
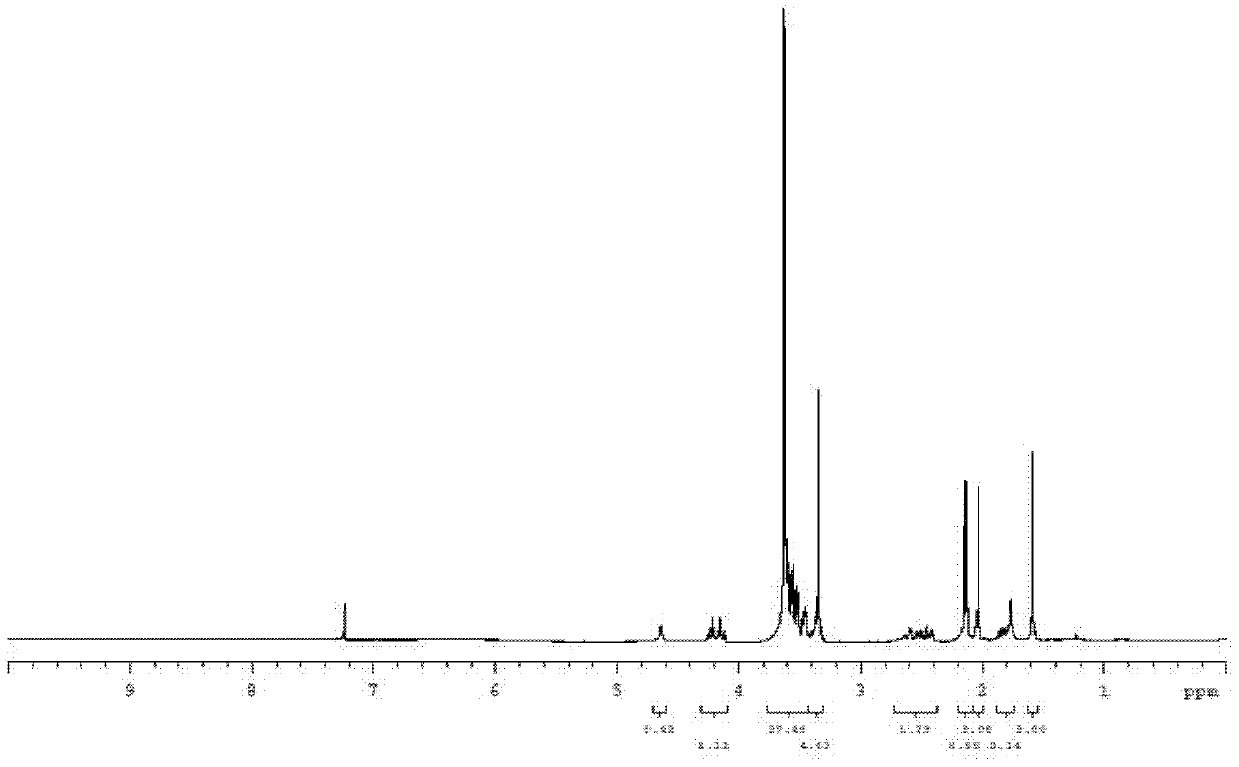
[0139] Example 1. 7-ethyl-10-hydroxycamptothecin R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid methoxypolyethylene Synthesis of glycol ester-6-succinate
[0140] The synthesis of the amphiphilic anticancer drug compound includes the following steps:
[0141] 1) Synthesis of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid methoxyheptapolyethylene glycol ester
[0142] The reaction formula is as follows:
[0143]
[0144] Experimental steps:
[0145] In a 50mL reaction flask, add 978mg (8mmol) 4-dimethylaminopyridine, 1.022mg (4mmol) 2-chloro-1-methylpyridinium iodide and 1021mg (3mmol) heptaethylene glycol monomethyl ether, With electromagnetic stirring, 751 mg (3 mmol) of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxyl was slowly added dropwise to the reaction solution. acid and 10 mL of a solution of N,N-dimethylformamide. The reaction was carried out under the protection of nitrogen at room temperature for 12 h, filtered to remove s...
Embodiment 2
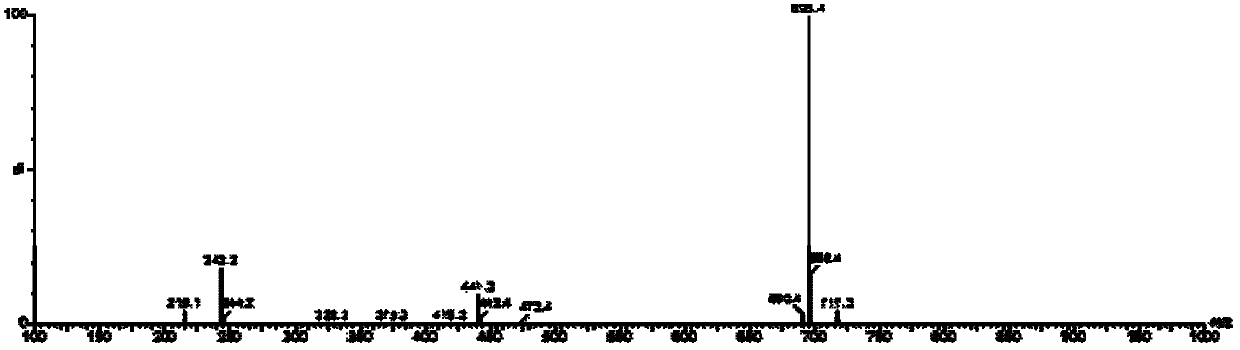
[0168] Example 2. 7-ethyl-10-hydroxycamptothecin (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid polyethylene glycol monomethyl ether Synthesis of Ester-6-Succinate
[0169] 1) Synthesis of (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid polyethylene glycol monomethyl ether ester (polyethylene glycol monomethyl ether) The average molecular weight of: Mn=550)
[0170] The reaction formula is
[0171]
[0172] Experimental steps:
[0173] In a 100mL reaction flask, add 2.20g (4mmol) polyethylene glycol monomethyl ether (average molecular weight is Mn=550), 1.45g (12mmol) 4-dimethylaminopyridine, 1.53g (6mmol) 2-chloro- 1-Methylpyridinium iodide and 30 mL of dioxane were stirred magnetically, and 1.00 g (4 mmol) of (±)-6-hydroxy-2,5,7,8-tetrakis was slowly added dropwise to the reaction solution. A solution of methylchroman-2-carboxylic acid and 30 mL dioxane. The reaction was carried out under the protection of nitrogen at room temperature for 12 h,...
Embodiment 3
[0196] Example 3. 7-ethyl-10-hydroxycamptothecin N-methoxyheptaethylene glycol amine R-(+)-6-hydroxy-2,5,7,8-tetramethylbenzodihydropyridine Synthesis of Pyran-2-carboxamide-6-succinate
[0197] The synthesis of the amphiphilic anticancer drug compound includes the following steps:
[0198] 1) Synthesis of N-methoxyheptapolyethylene glycol amine group R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxamide
[0199] The reaction formula is as follows:
[0200]
[0201] Experimental steps:
[0202] In a 50mL reaction flask, add 825mg (4mmol) N,N'-dicyclohexylcarbodiimide (DCC), 1018mg (3mmol) methoxyheptapolyethylene glycol amine, 751mg (3mmol) R-(+ )-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid and 10 mL of N,N-dimethylformamide. Electromagnetic stirring, react for 12h at room temperature and under the protection of nitrogen, remove the solid material by filtering, concentrate the filtered solution to 10mL with a rotary evaporator, and separate the column laye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com