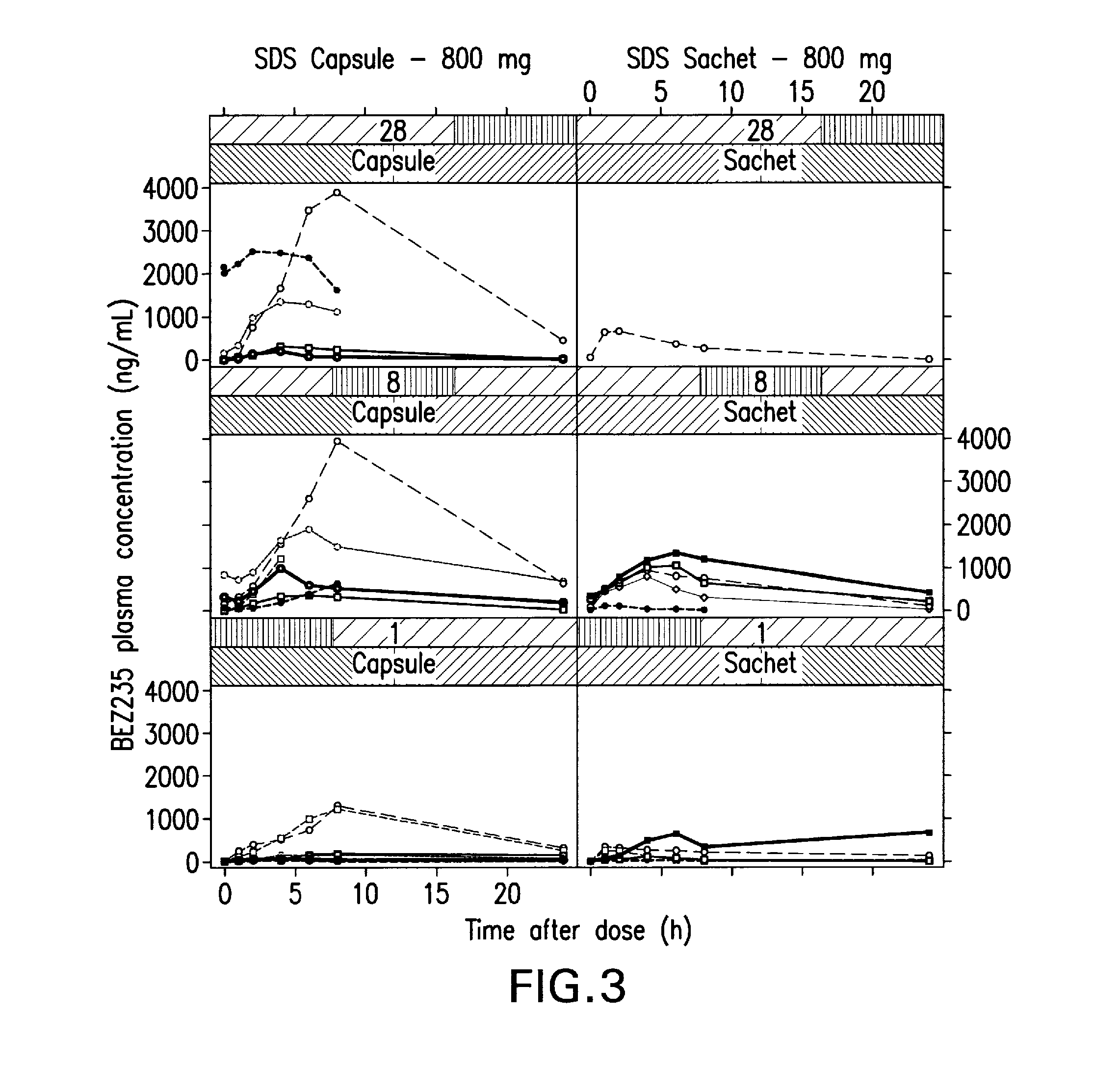
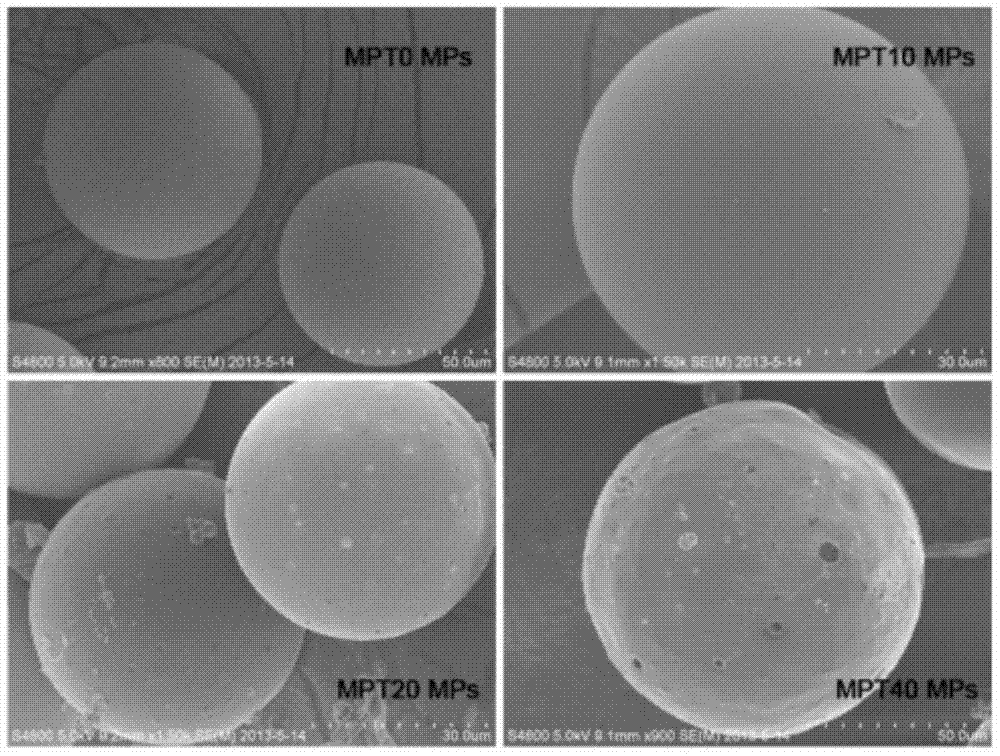
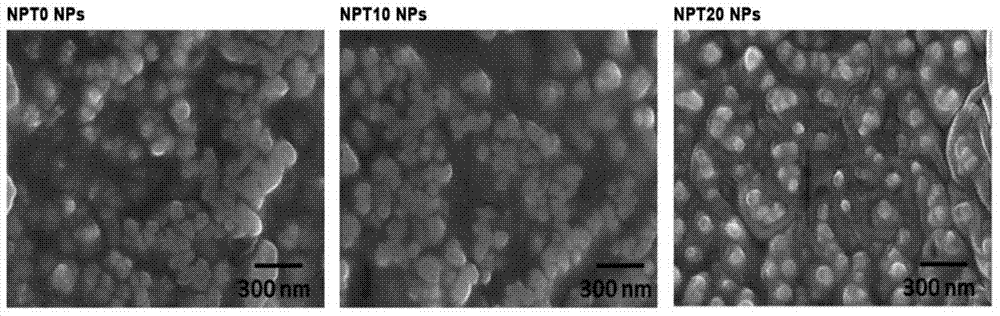
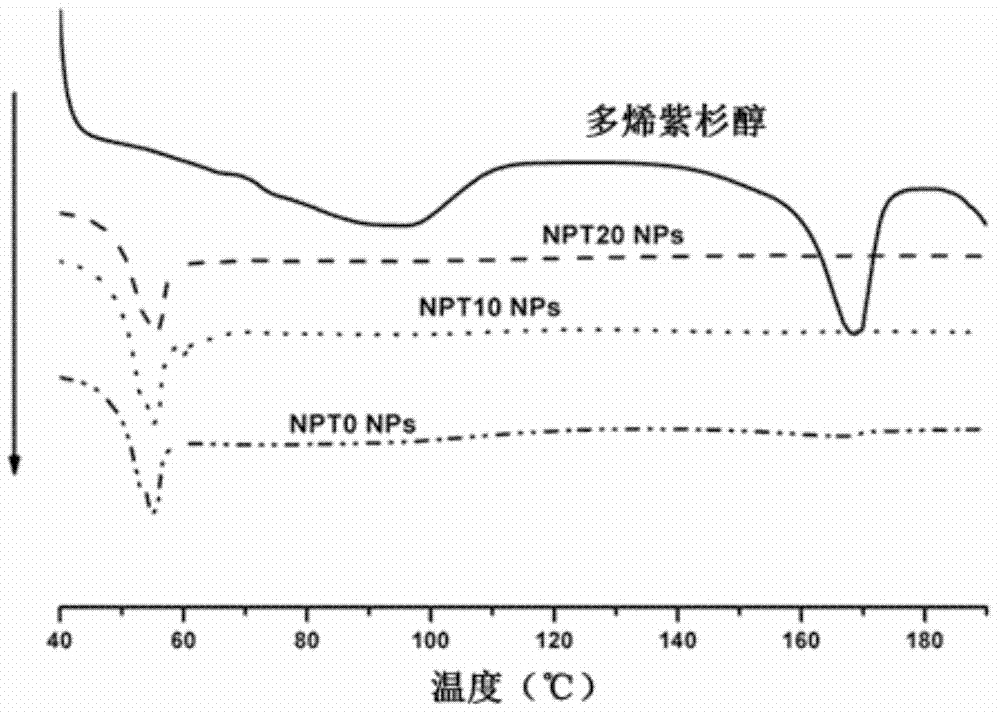
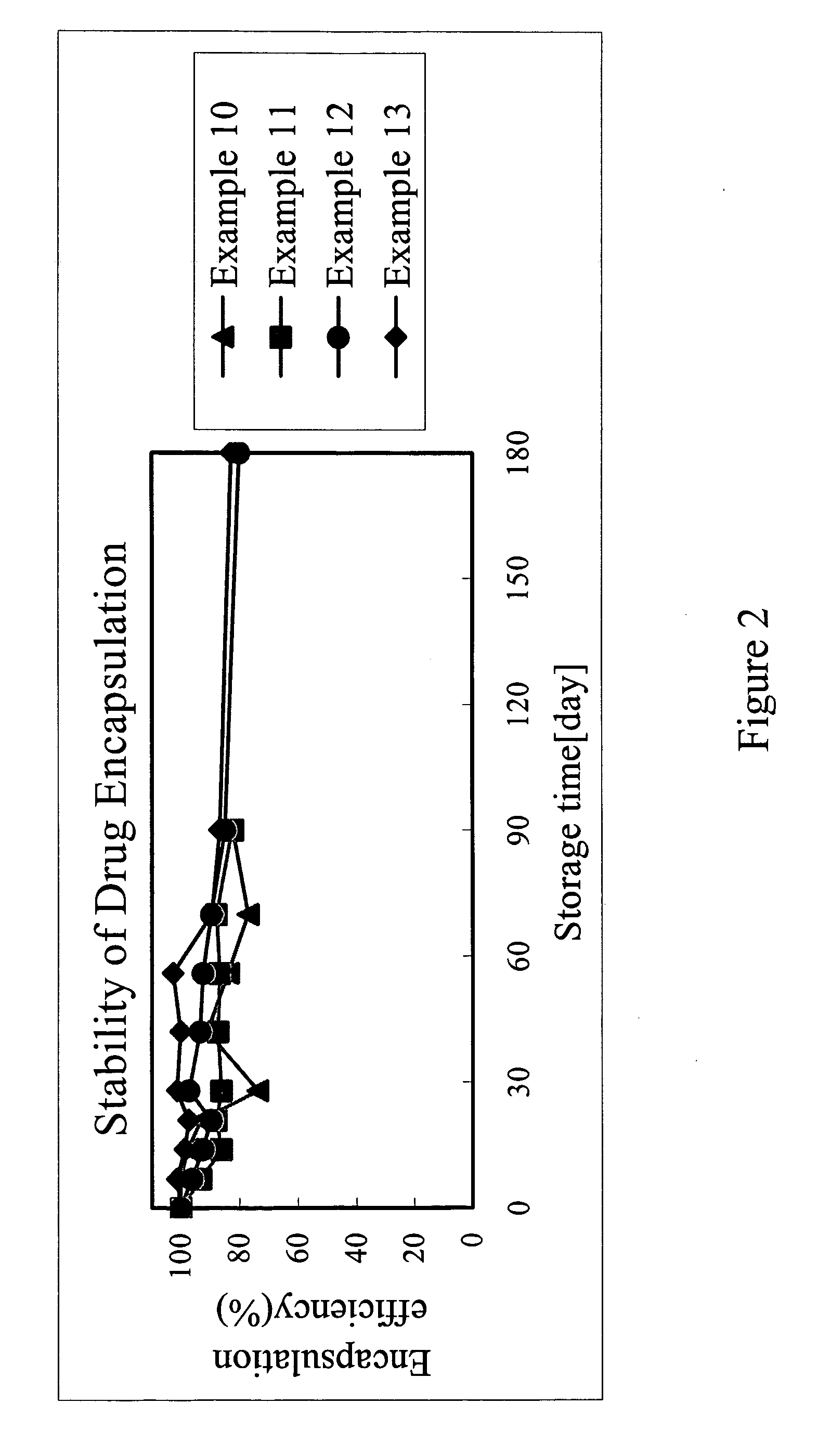
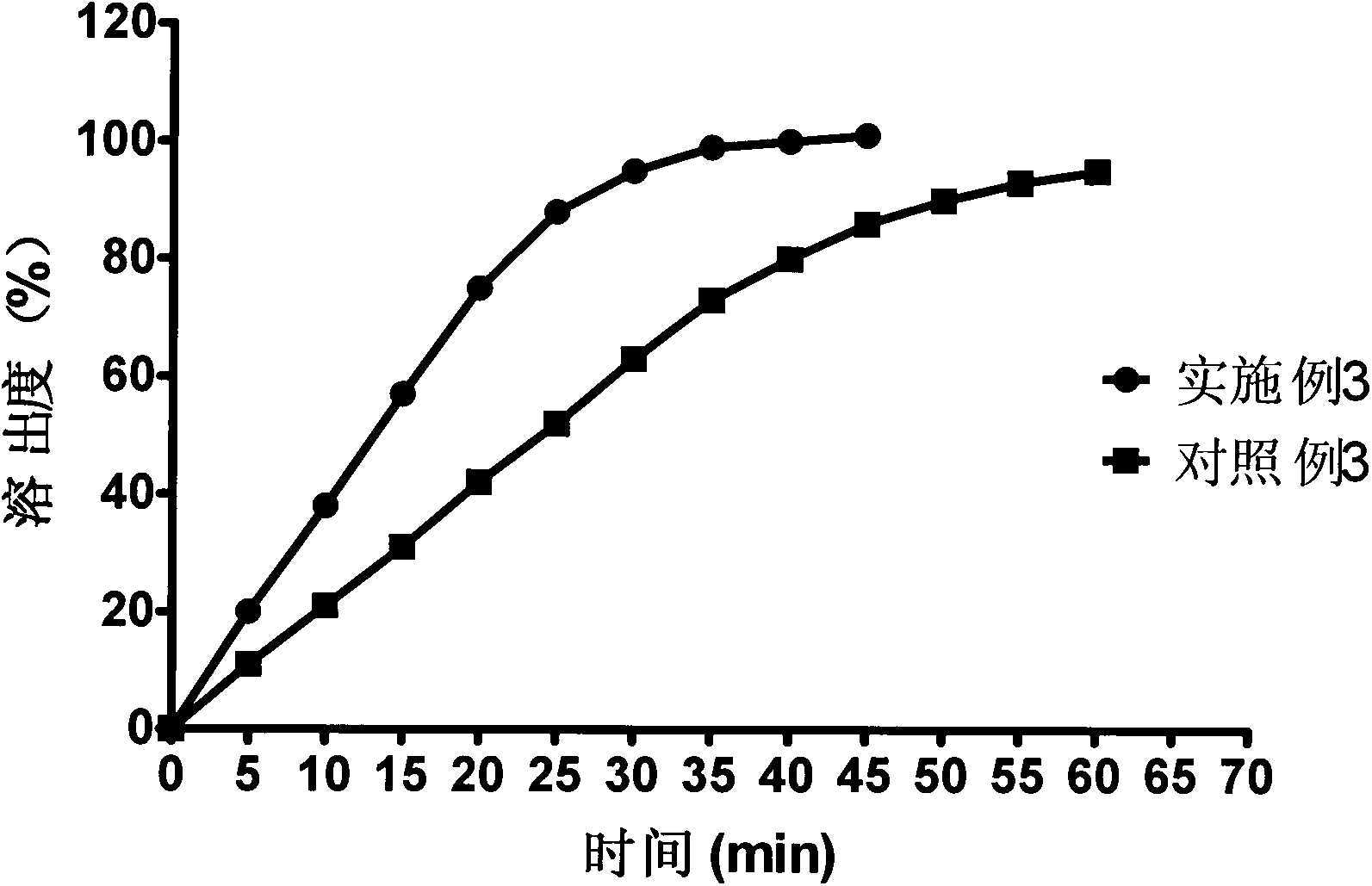
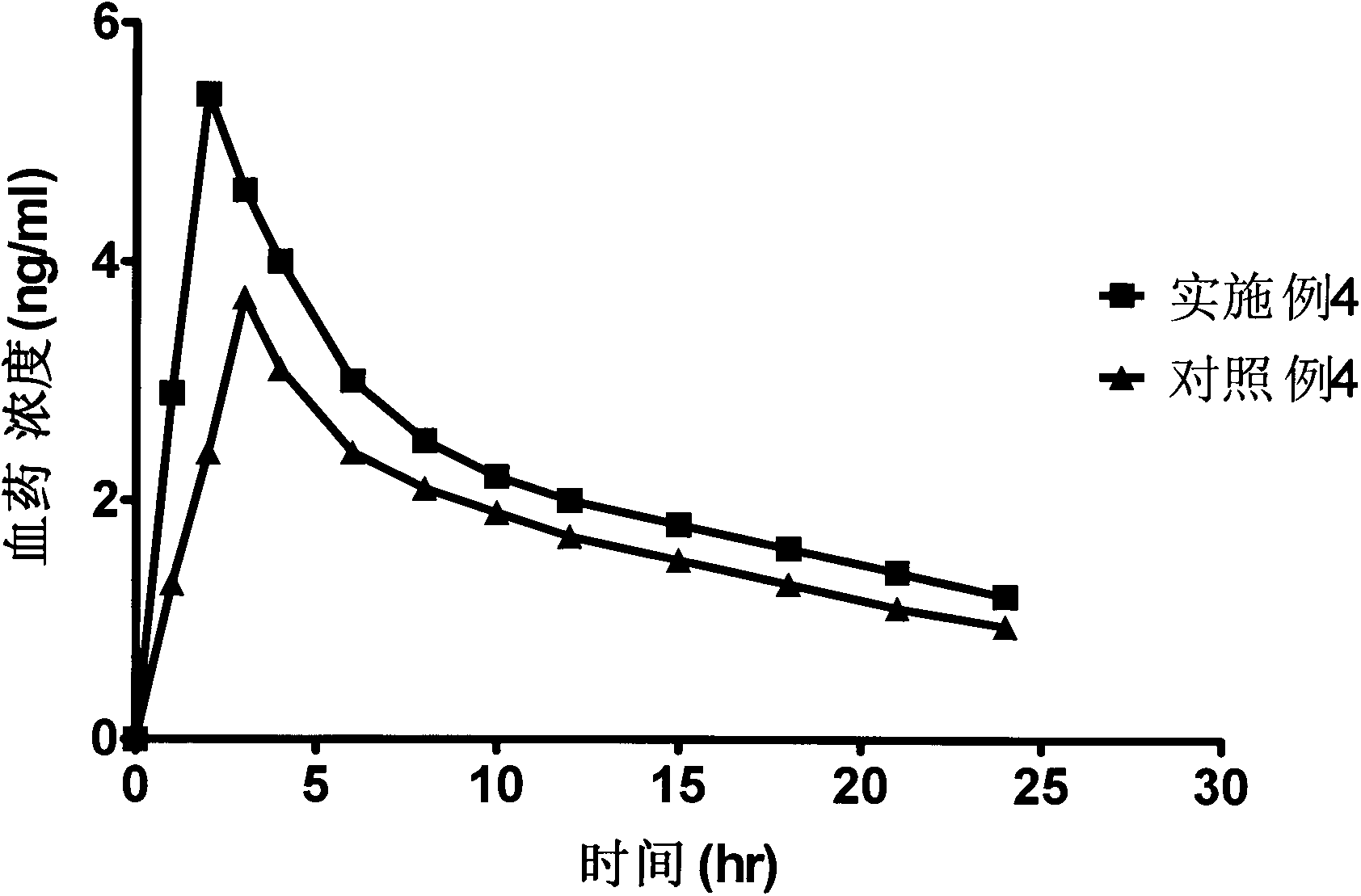
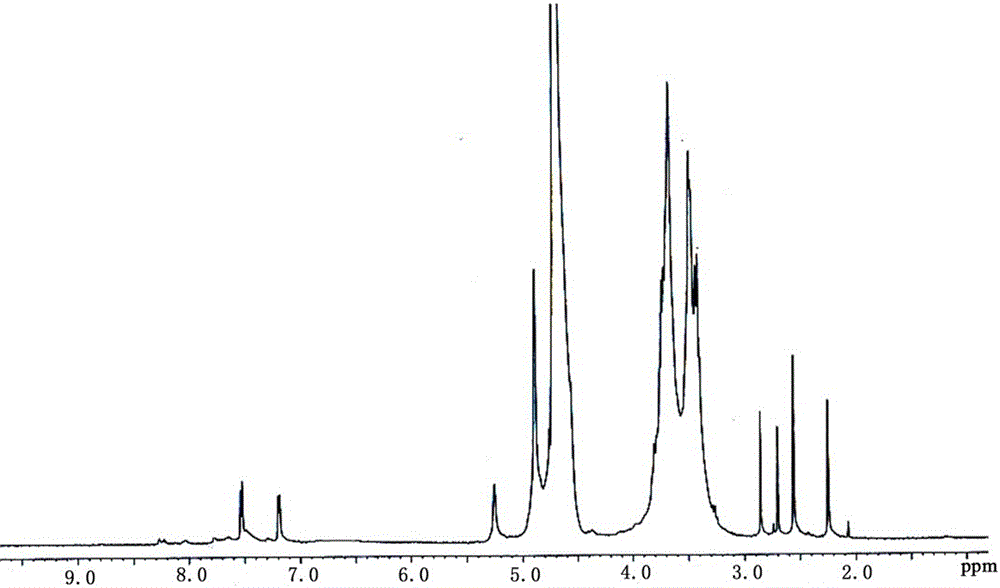
Patents
Literature
46 results about "Vitamin E-TPGS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vitamin E TPGS, a water soluble derivative of natural Vitamin E, is a multirole excipient for pharmaceutical drug delivery innovation. Vitamin E TPGS is a non-ionic surfactant used as:
Aqueous emulsions of lipophile solubilized with vitamin E TPGS and linoleic acid
Disclosed herein is an aqueous emulsion. The lipid phase of the emulsion includes a blend of a therapeutically effective concentration of a lipophile, a concentration of Vitamin E TPGS, and a concentration of linoleic acid. The presence of linoleic acid increases the solubilizing affect of Vitamin E TPGS on the lipophile and thus reduces the amount of Vitamin E TPGS that would otherwise be required in the aqueous emulsion.
Owner:CALLION PHARMA LLC
Vitamin E TPGS fluid concentrate comprising a low percentage of water
A liquid Vitamin E TPGS concentrate composition is provided, the concentrate having a significantly higher percentage of TPGS per unit volume than traditional liquid TPGS formulations while providing the TPGS in a liquid form that can be used in softgels or mixed with water to produce desired concentrations for commercial use in supplements, beverages, pharmaceutical preparations, etc.
Owner:JANDZINSKI ROBERT +3
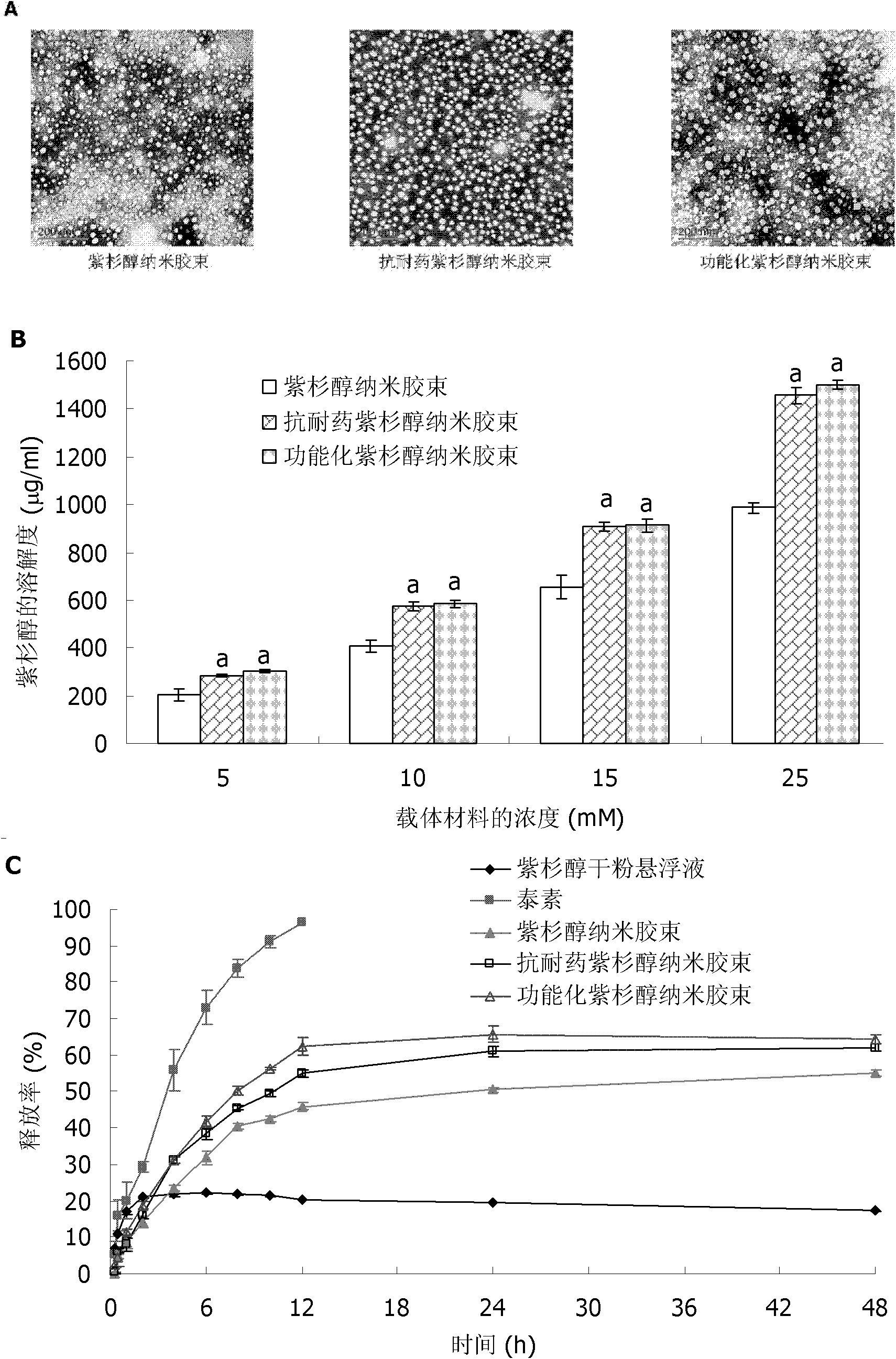
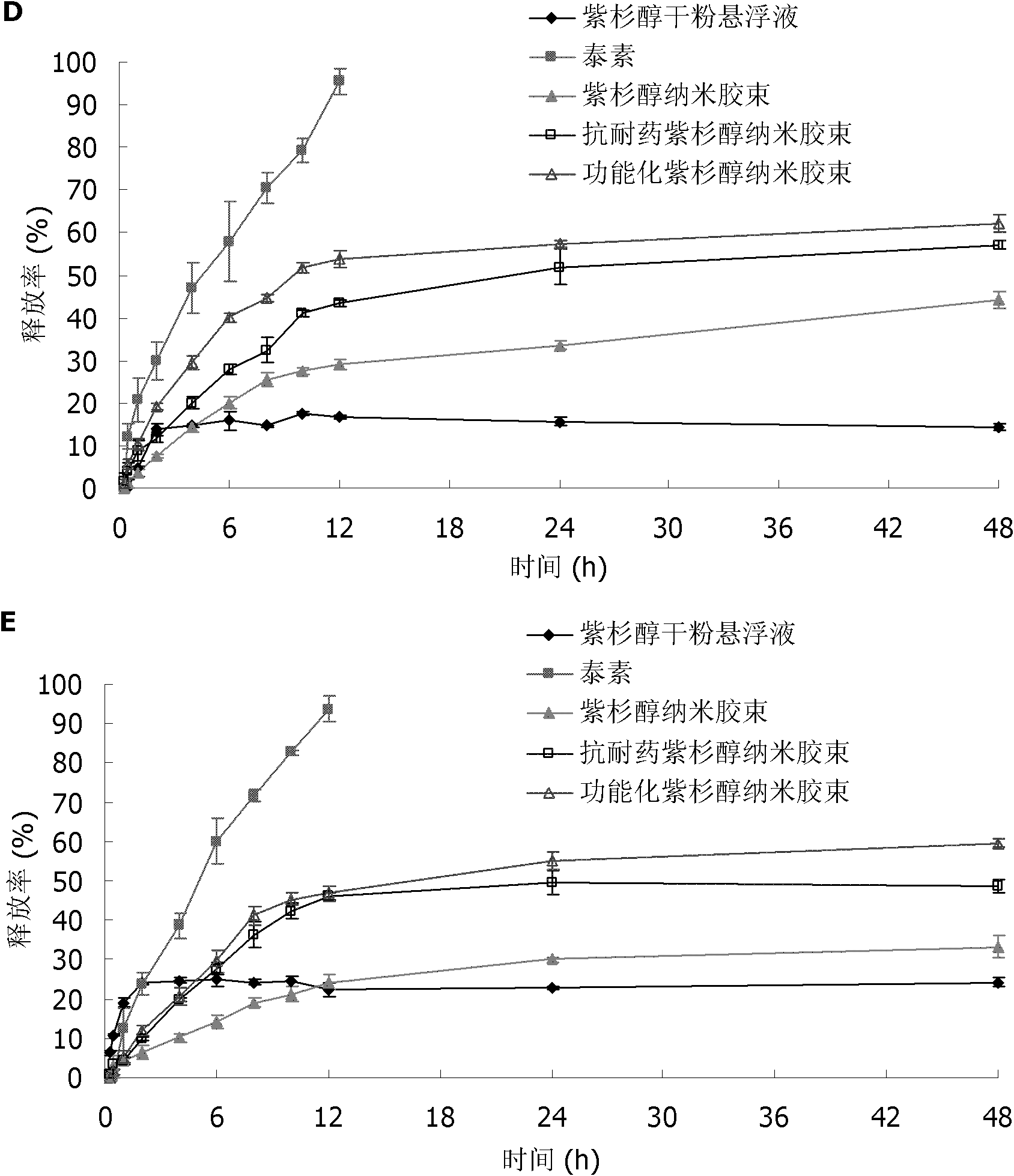
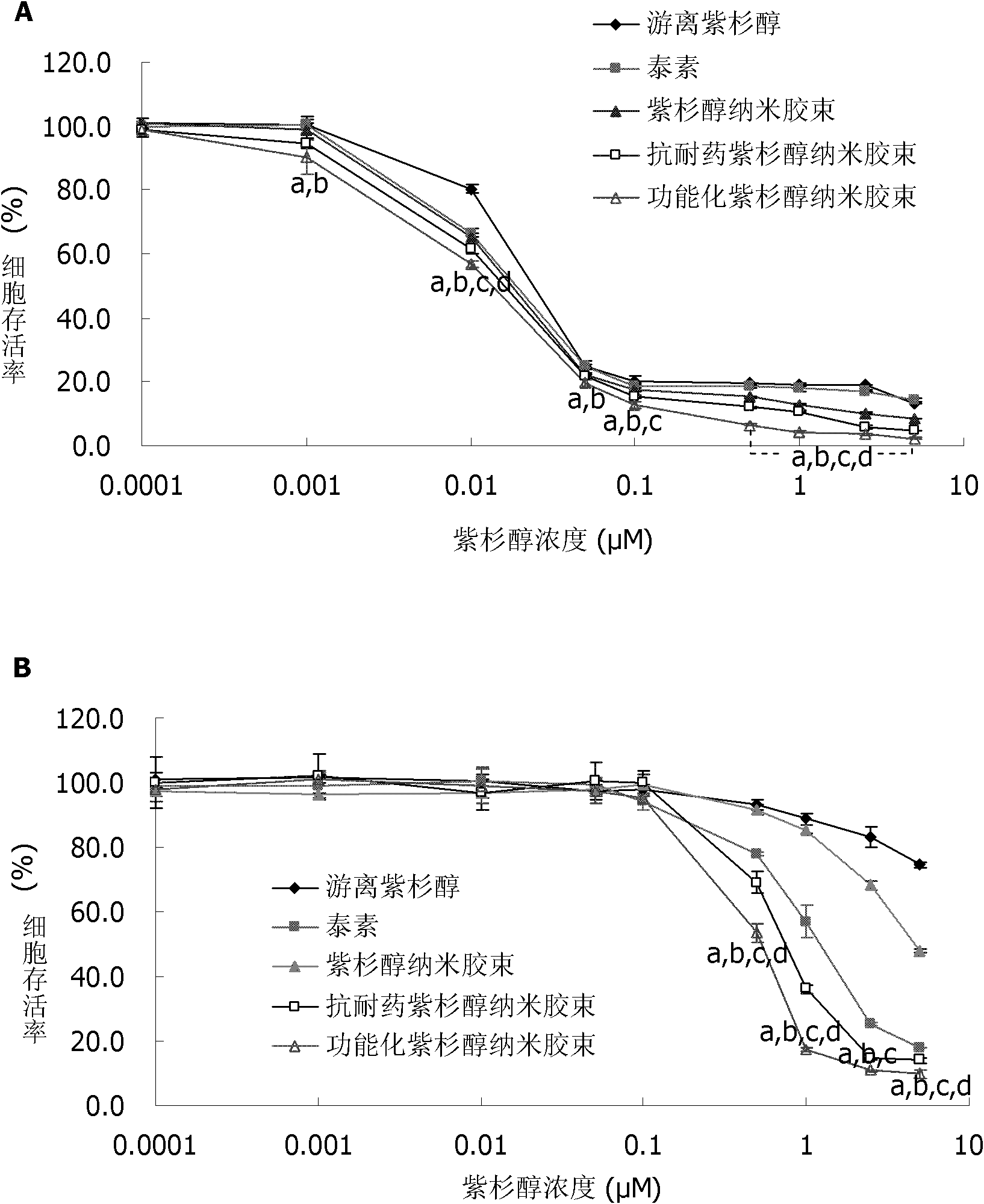
Paclitaxel nano micelle and application thereof
InactiveCN102133172AIncreased intracellular uptakeEnhanced inhibitory effectOrganic active ingredientsPharmaceutical delivery mechanismDequalinium chlorideEthanolamine synthesis
The invention discloses paclitaxel nano micelle and an application thereof. The paclitaxel nano micelle is prepared from the following components shown in the following (1), (2) or (3): (1) a carrier and paclitaxel, wherein the carrier is a mixture of polyethylene glycol-distearoyl phosphatidyl ethanolamine (PEG-DSPE), D-alpha-tocopherol polyethylene glycol 1000 succinate (TPGS) and dequalinium chloride; (2) a carrier and the paclitaxel, wherein the carrier is a mixture of the PEG-DSPE and the TPGS; and (3) a carrier and the paclitaxel, wherein the carrier is the PEG-DSPE. After the functionalized paclitaxel nano micelle disclosed by the invention is orally taken, the paclitaxel can penetrate through a barrier of gastrointestinal tract, thus the multi-drug resistance (MDR) of drug-resistant breast cancer is overcome. The oral formulation of the functionalized paclitaxel nano micelle disclosed by the invention can avoid the anaphylactic response, can be applied to numerous cancer patients and is also suitable for MDR tumors at the same time, thereby having great advantage compared with the traditional intravenous injection formulation.
Owner:PEKING UNIV
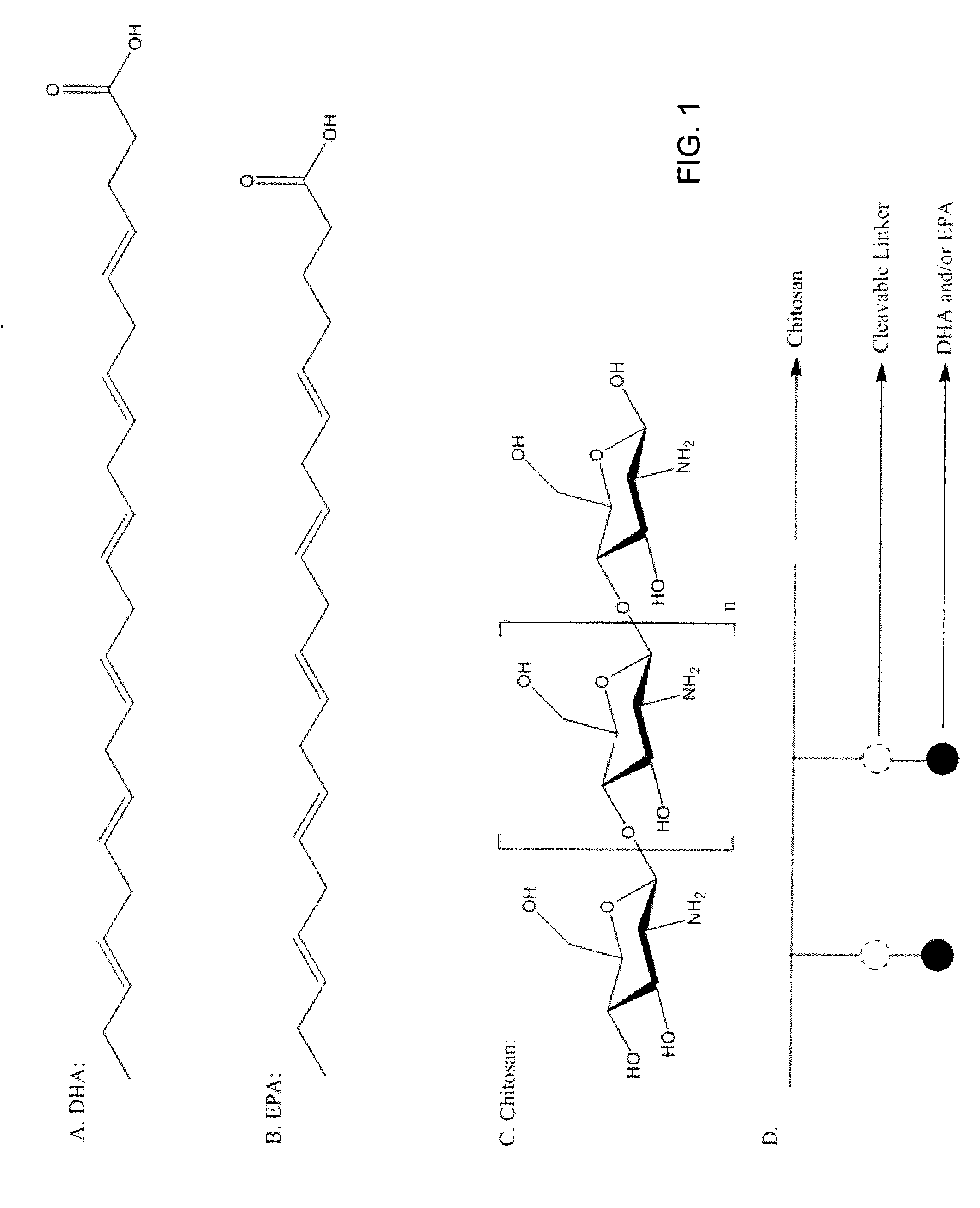
Nanoformulation of vitamin d derivatives and/or vitamin d metabolites
A nanoformulation that includes loaded nanoparticles. Each nanoparticle includes a modified chitosan polymer encapsulating at least one vitamin D derivative, at least one vitamin D metabolite, or combinations thereof. The modified chitosan polymer includes chitosan covalently linked to at least one entity selected from the group consisting of fatty acids (omega-3-fattay acids), amino acids, deoxycholic acid, alginate, arginine-alginate, hyaluronic acid, collagen, collagen-hydroxyapatite, poly(lactic-co-glycolic acid) (PLGA), and combinations thereof. A structure includes a medium and the nanoformulation, wherein the nanoparticles are dispersed in the medium. A method of using the nanoformulation to treat a disorder and improve efficacy of current therapies where resistance develop in a patient includes administering to the patient a therapeutically effective amount of the nanoformulation for treating the disorder. A nano-cosmetic formulation, comprising a cosmetic includes the nanoformulation, wherein the modified chitosan polymer encapsulates the at least one vitamin D derivative, and wherein the at least one vitamin D derivative encompasses 0.1 to 20.0 wt % of the nano-cosmetic formulation's total weight.
Owner:MOUSA SHAKER A
Nano-particle containing docetaxel and vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) and preparation method thereof
InactiveCN103751107AEfficient targetingGood chemical stabilityOrganic active ingredientsPowder deliverySide effectDocetaxel
The invention discloses a nano-particle containing docetaxel and vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) and a preparation method thereof. The nano-particle containing docetaxel and vitamin E TPGS comprises the following components in parts by weight: 2-10 parts of docetaxel, 1-15 parts of vitamin E TPGS, 20-65 parts of albumin, 5-50 parts of cryoprotectant, and 2-20 parts of acidic buffer salt. Relative to an existing docetaxel commercial preparation, the nano-particle containing docetaxel and vitamin E TPGS is good in drug entrapping performance, small in toxic and side effect, high in stability, good in anti-tumor curative effect and convenient to administer; the preparation method is simple, and the production cost is low.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Paclitaxel entrapped polymeric micelle for treating tumors, and preparation method thereof
InactiveCN104997758AHigh drug loadingReduce adverse reactionsOrganic active ingredientsPharmaceutical non-active ingredientsSolubilitySide effect
The invention belongs to the technical field of biomedicines, and relates to a paclitaxel entrapped polymeric micelle for treating tumors, and a preparation method thereof. The preparation method comprises the following steps: preparing paclitaxel PEG-PLA / Vitamin E-TPGS mixed micelle through adopting a film dispersion technology, and entrapping paclitaxel into the hydrophobic core of the mixed micelle by using the mixed micelle composed of PEG-PLA and Vitamin E-TPGS as a carrier. The prepared paclitaxel paclitaxel PEG-PLA / Vitamin E-TPGS mixed micelle can effectively increase the solubility of paclitaxel and substantially improves the paclitaxel load of a micelle system to reach 35% (w / w), and Vitamin E-TPGS in the mixed micelle system has a P-gp efflux inhibition biologic function, and can efficiently reverse the multidrug resistance of tumors in order to improve the tumor treatment effect. The mixed micelle does not contain Tween80 or ethanol; and compared with commercial paclitaxel injections, the above preparation provided by the invention has the advantages of no allergy of commercial preparations, reduction of toxic side effects, and increase of the safety of clinic application.
Owner:FUDAN UNIV
Nano-micelle drug carrier based on vitamin E derivative, nano-micelle drug composition as well as preparation method and application of nano-micelle drug composition
ActiveCN107019670AEnhanced inhibitory effectLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsBiocompatibility TestingTocopherol succinate
The invention provides a nano-micelle drug carrier based on a vitamin E derivative, a nano-micelle drug composition as well as a preparation method and application of the nano-micelle drug composition. The nano-micelle drug carrier based on the vitamin E derivative is a nano-micelle formed by D-alpha-tocopherol polyethylene glycol 1000 succinate, D-alpha-tocopherol polyethylene glycol 2000 succinate and alpha-tocopherol succinate; and the carrier entraps a hydrophobic anti-tumor drug to obtain the nano-micelle drug composition. The D-alpha-tocopherol polyethylene glycol 1000 succinate, D-alpha-tocopherol polyethylene glycol 2000 succinate and alpha-tocopherol succinate are matched with one another and cooperate, so that the formed nano-micelle is good in stability, is small and uniform in grain size, improves drug encapsulation efficiency, has relatively good P-glycoprotein inhibiting ability, has good anti-tumor effect, is small in toxicity to normal cells and has good biocompatibility. The preparation method is simple and is wide in application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Pharmaceutical compositions
InactiveUS20130245061A1Improve bioavailabilityBiocideOrganic chemistryPolyethylene oxideOral medication
A pharmaceutical composition for the oral administration of a therapeutic compound of formula (I), comprising granules that comprise at least therapeutic compound of formula (I) or a tautomer thereof, or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; at least one non-ionic surfactant that is Vitamin E-TPGS in an amount ranging from about 15 to about 80% by weight of the composition; and at least one a dissolution enhancing agent selected from polyethylene glycol, polyethylene oxide, and any combination of the foregoing; processes for making such pharmaceutical compositions; a kit comprising such pharmaceutical composition and the instructions for administration thereof; and related uses and methods of treatment.
Owner:NOVARTIS AG
Application of vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) in preparing porous drug carrier particles
InactiveCN103751787AEfficient collectionLow toxicityPharmaceutical non-active ingredientsGranular deliveryPorositySide effect
The invention discloses application of vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) in preparing porous drug carrier particles, a method for preparing porous drug carrier particles, and the porous drug carrier particles. The method comprises the following steps: dissolving vitamin E TPGS, medicines and organic macromolecular polymer, such as PLGA (poly(lactic-co-glycolic acid)), in an organic solvent; emulsifying, removing the organic solvent in a volatizing manner to obtain the drug-loaded porous drug carrier particles with a porous structure on the surface. The porous drug carrier particles have the characteristics of small toxicity, high target, high encapsulation rate, porosity and drug in-vitro release acceleration, can be used for inhibiting P-gp induced drug transportation due to TPGS, so that the multi-drug resistance effect of an administrated object can be inhibited; furthermore, the porous drug carrier particles are utilized in administration, and the drug utilization is high, so that the dosage can be effectively reduced, and side effects can be further reduced.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Amphiphilic anti-cancer drug compound modified by water-soluble vitamin E derivative, preparation, preparation method and application for compound
InactiveCN102491981AOrganic active ingredientsGroup 5/15 element organic compoundsChemical compoundDrug compound
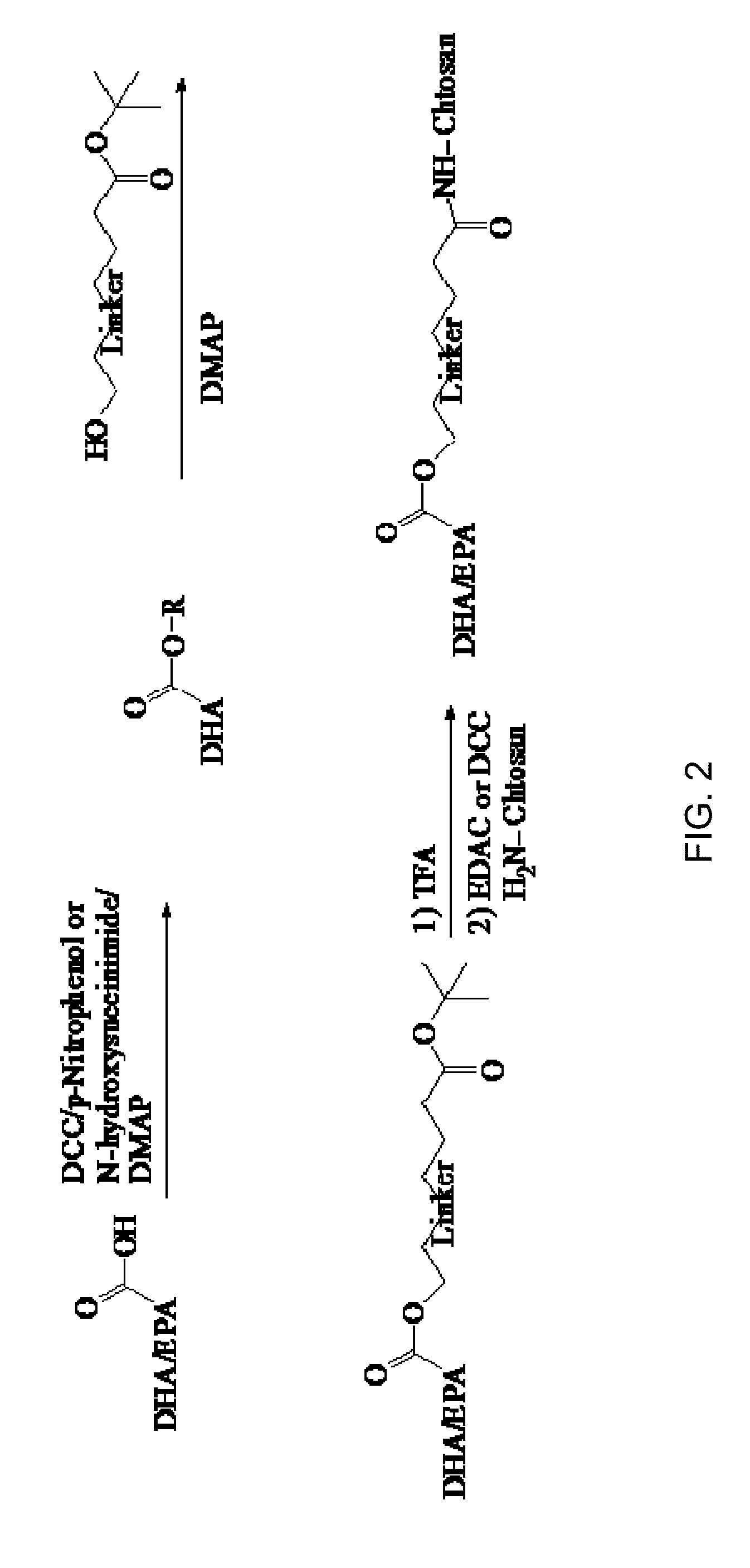
The invention discloses an amphiphilic anti-cancer drug compound modified by a water-soluble vitamin E derivative. The amphiphilic anti-cancer drug compound has the structure of the following formula I or II. The anti-cancer drug active part camptothecin or camptothecin derivative, and amphiphilic part water-soluble vitamin E alkoxy polyethylene glycol ester or amide are covalently bonded by linking groups to form the amphiphilic anti-cancer drug compound. The invention further relates to a preparation, a preparation method and an application for the medicine compound.
Owner:NANJING MEIXINING MEDICAL TECH
Cyclosporine emulsion and the preparing method
The present invention provides one kind of self-emulsified ciclosporin preparation and its preparation process. The self-emulsified ciclosporin preparation contains ciclosporin 5-25 wt%, vitamin E-TPGS 25-70 wt% and Pharmasolve 5-50 wt%. It has high stability, high medicine concentration, great capacity of self emulsifying, high bioactivity and easy taking, and may be used as orally taken liposoluble medicine.
Owner:SHANGHAI KAIZHAO PHARMA TECH
Water soluble compositions comprising purified cannabinoids
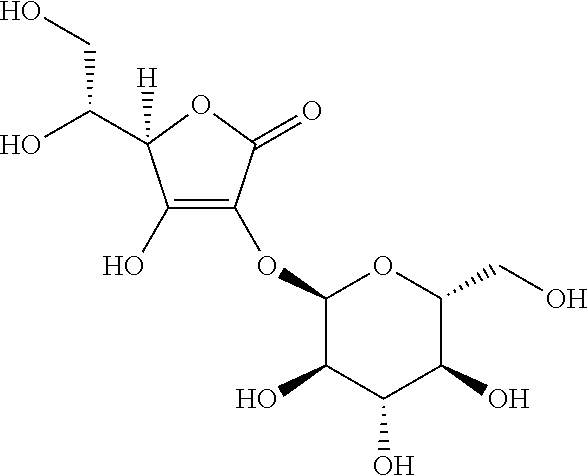
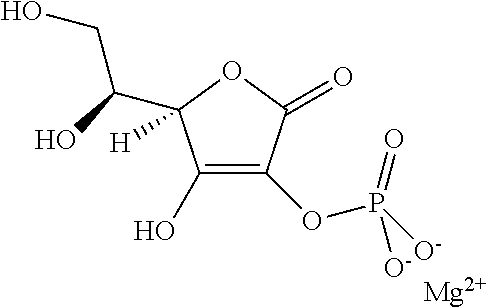
InactiveCN110177543AHydrocarbon active ingredientsHydroxy compound active ingredientsWater solubleVitamin
This disclosures relates to new compositions and methods for making cannabinoid formulations. In one embodiment, this disclosure provides water soluble compositions comprising a first purified cannabinoid and Vitamin E TPGS. In one embodiment, the disclosure herein comprises a method of making powders comprising heatings material to a first temperature and a second temperature.
Owner:凯诺比生长公司
Aqueous emulsions of lipophile solubilized with vitamin E TPGS and linoleic acid
Disclosed herein is an aqueous emulsion. The lipid phase of the emulsion includes a blend of a therapeutically effective concentration of a lipophile, a concentration of Vitamin E TPGS, and a concentration of linoleic acid. The presence of linoleic acid increases the solubilizing affect of Vitamin E TPGS on the lipophile and thus reduces the amount of Vitamin E TPGS that would otherwise be required in the aqueous emulsion.
Owner:CALLION PHARMA LLC
Preparation method and application of vitamin E molecularly imprinted polymer
InactiveCN109280125AEasy to identifyImprove adsorption capacityOther chemical processesSpecific adsorptionOrganic solvent
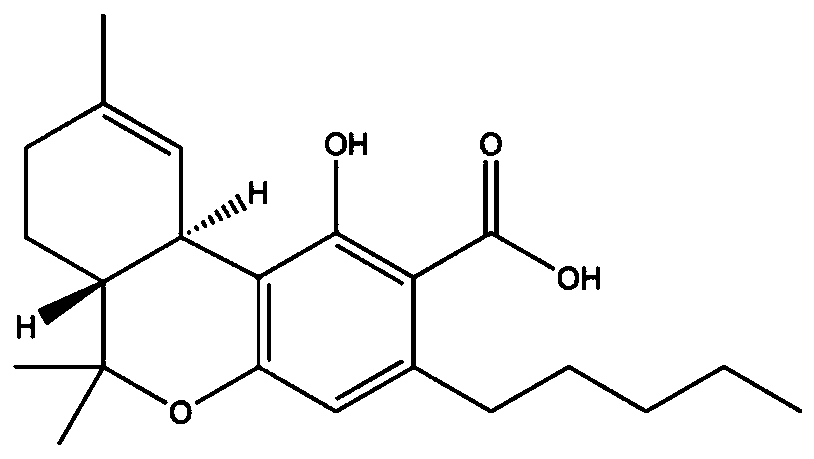
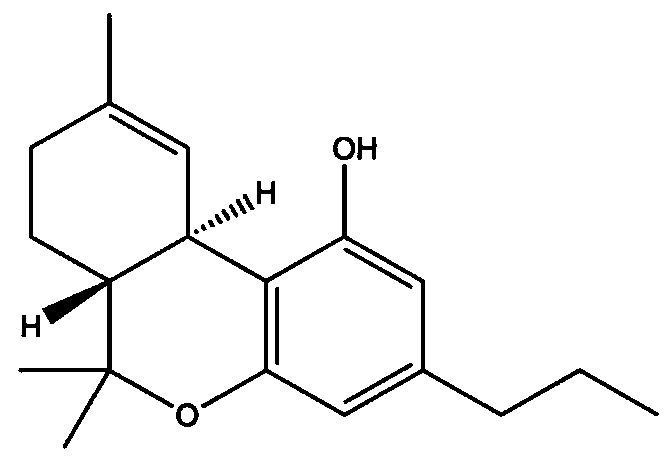
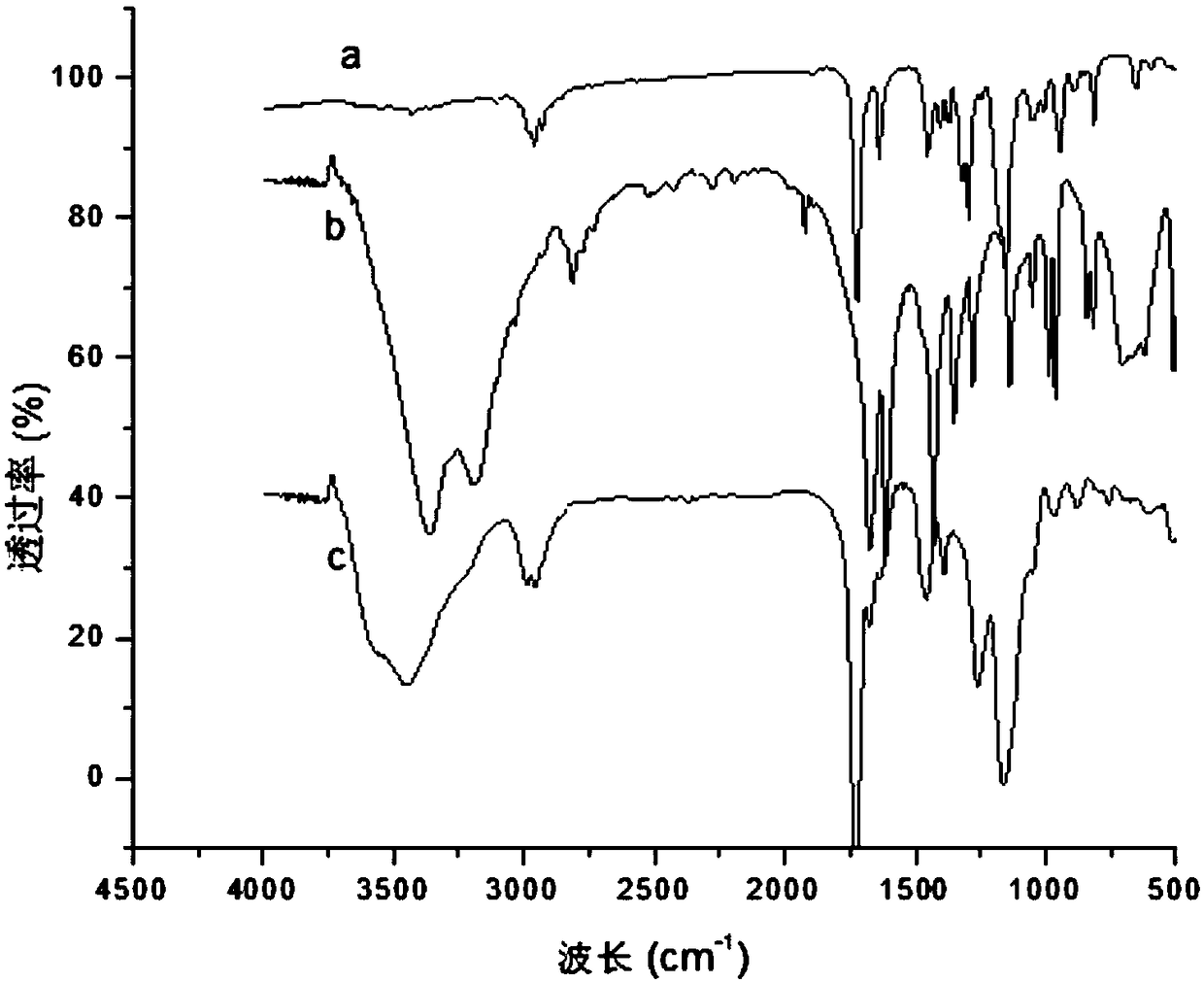
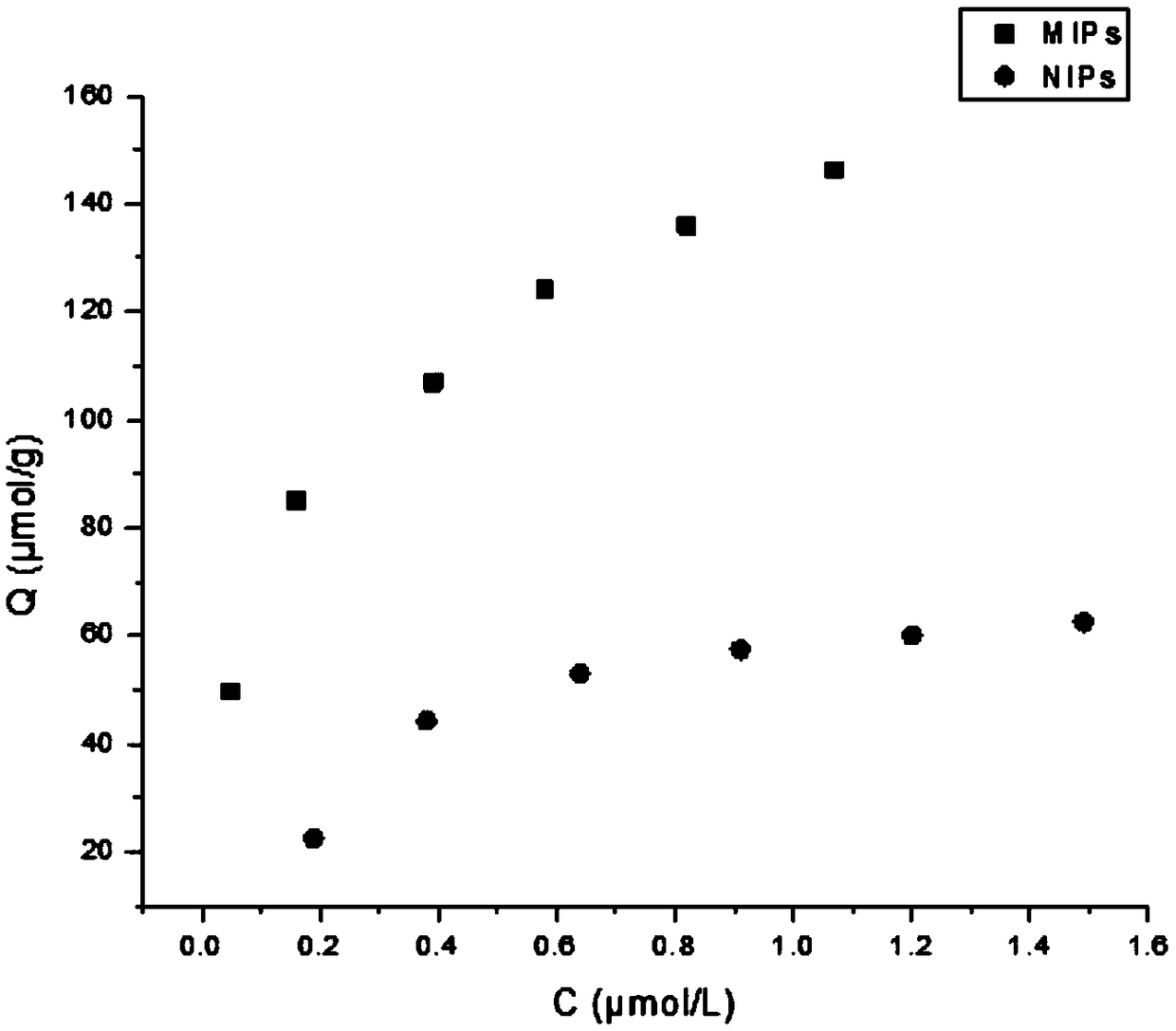
The invention relates to a preparation method and application of vitamin E molecularly imprinted polymer. According to the preparation method, acetone is adopted as an organic solvent, vitamin E, acrylamide, ethylene glycol dimethacrylate and azodiisobutyronitrile are adopted as raw materials, and the molecularly imprinted polymer is obtained through a reaction at 60-70 DEG C under the conditionsof oxygen isolation. It is shown through experiment that the polymer has specific adsorption properties for vitamin E, and vitamin E can be identified precisely and separated from a complex system, and the successful synthesis of the molecularly imprinted polymer is of great significance to the industrial separation and purification of vitamin E.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
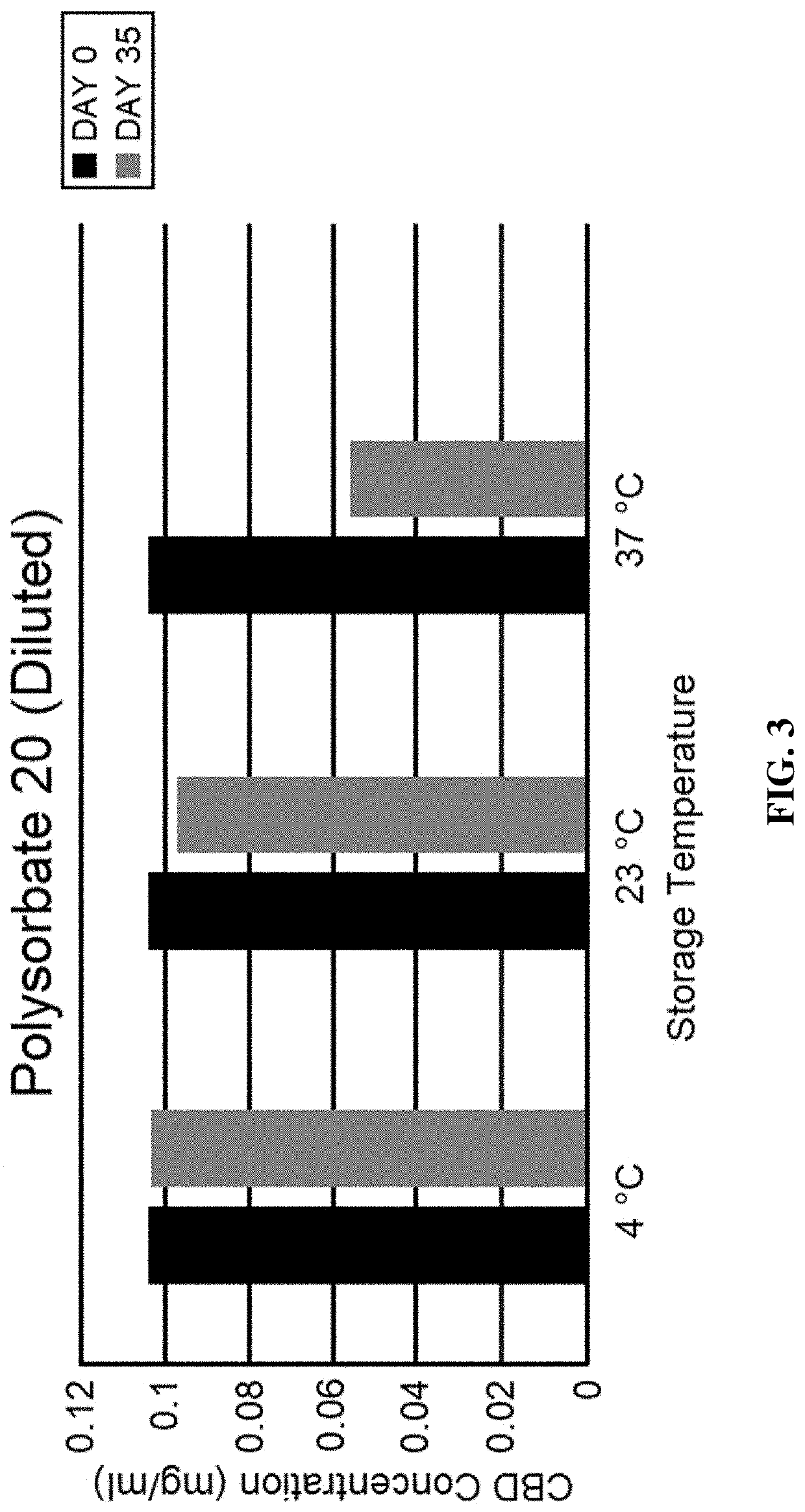
Transdermal delivery of cannabidiol with other active moieties including cannabinoids
InactiveUS20180078512A1Hydroxy compound active ingredientsPharmaceutical non-active ingredientsTransdermal patchReservoir type
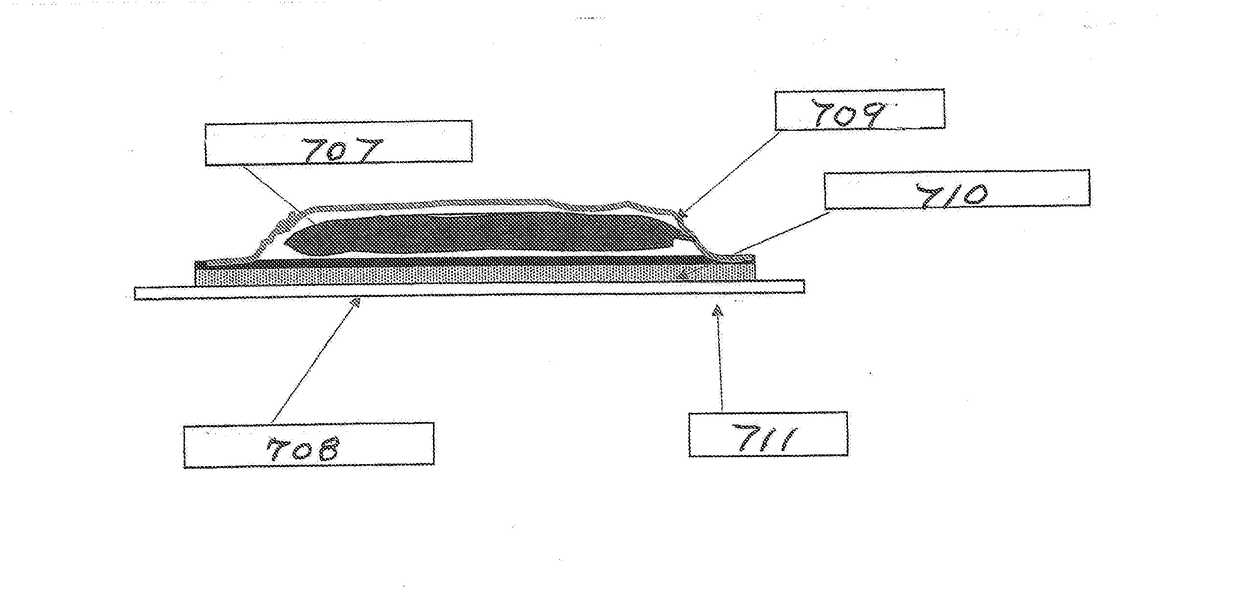
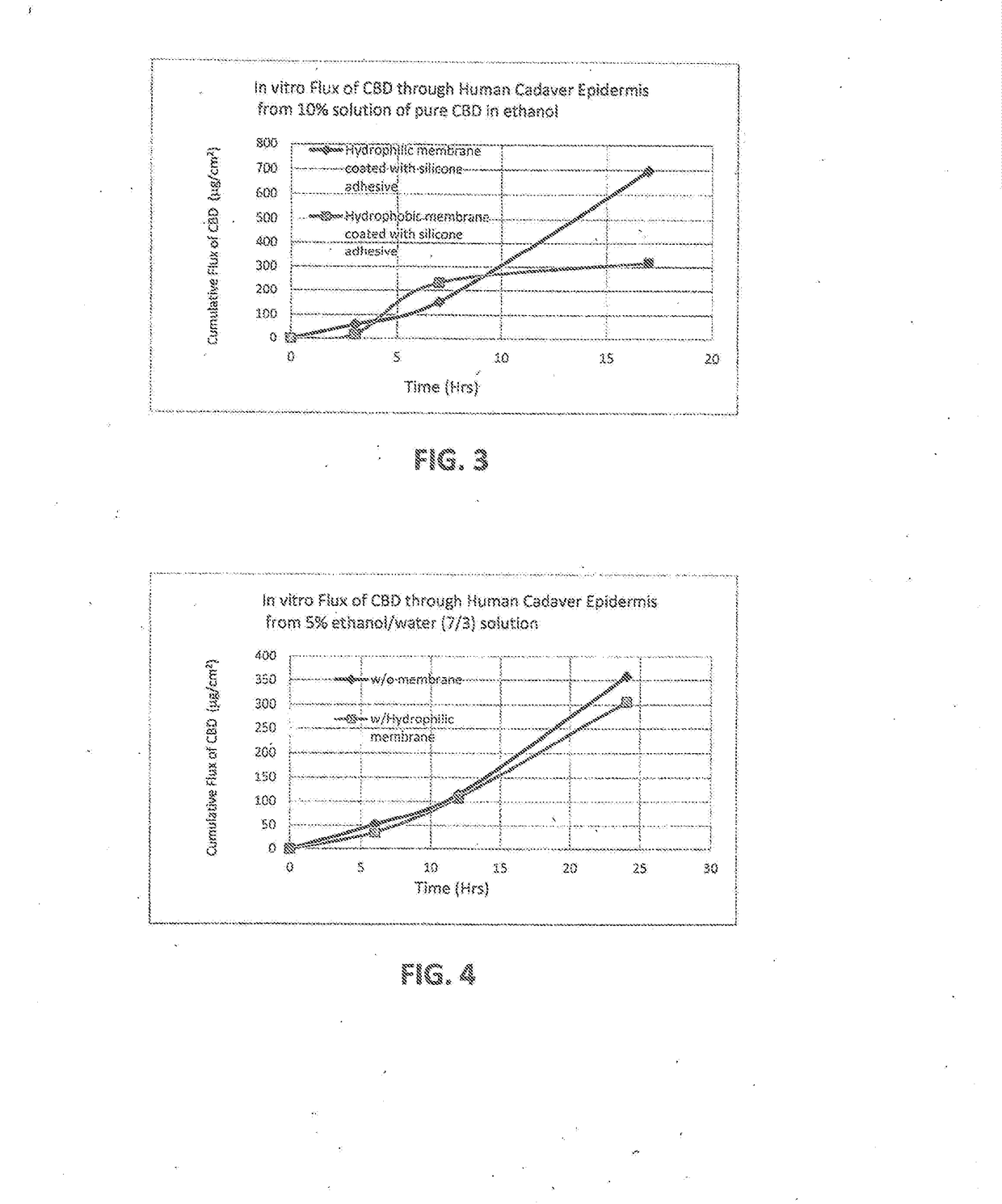
Transdermal delivery devices for the delivery of cannabidiol (CBD) and related moieties are shown and described. In a reservoir-patch design, a microporous, hydrophilic membrane and a backing define a reservoir that houses a mixture of CBD, a polar liquid, and a gelling agent, along with other moieties that seem to be enhanced or bioavailability of increased by the same. The hydrophilic membrane is coated with an amine-compatible silicone skin adhesive. In a monolithic design, a release liner is coated with a mixture of CBD and a PIB or amine-compatible silicone skin adhesive laminated to the backing material. In using CBD as a pure compound or as one to combine with other moieties, the invention is able to control delivery better than the prior art. Construction of the Transdermal patch of reservoir type is also taught, which enables things such as Vitamin B 12 to become part of the complex deliverable by the inventions.
Owner:REMY BIOSCIENCES INC
Tanshinone lipidosome and preparation method thereof
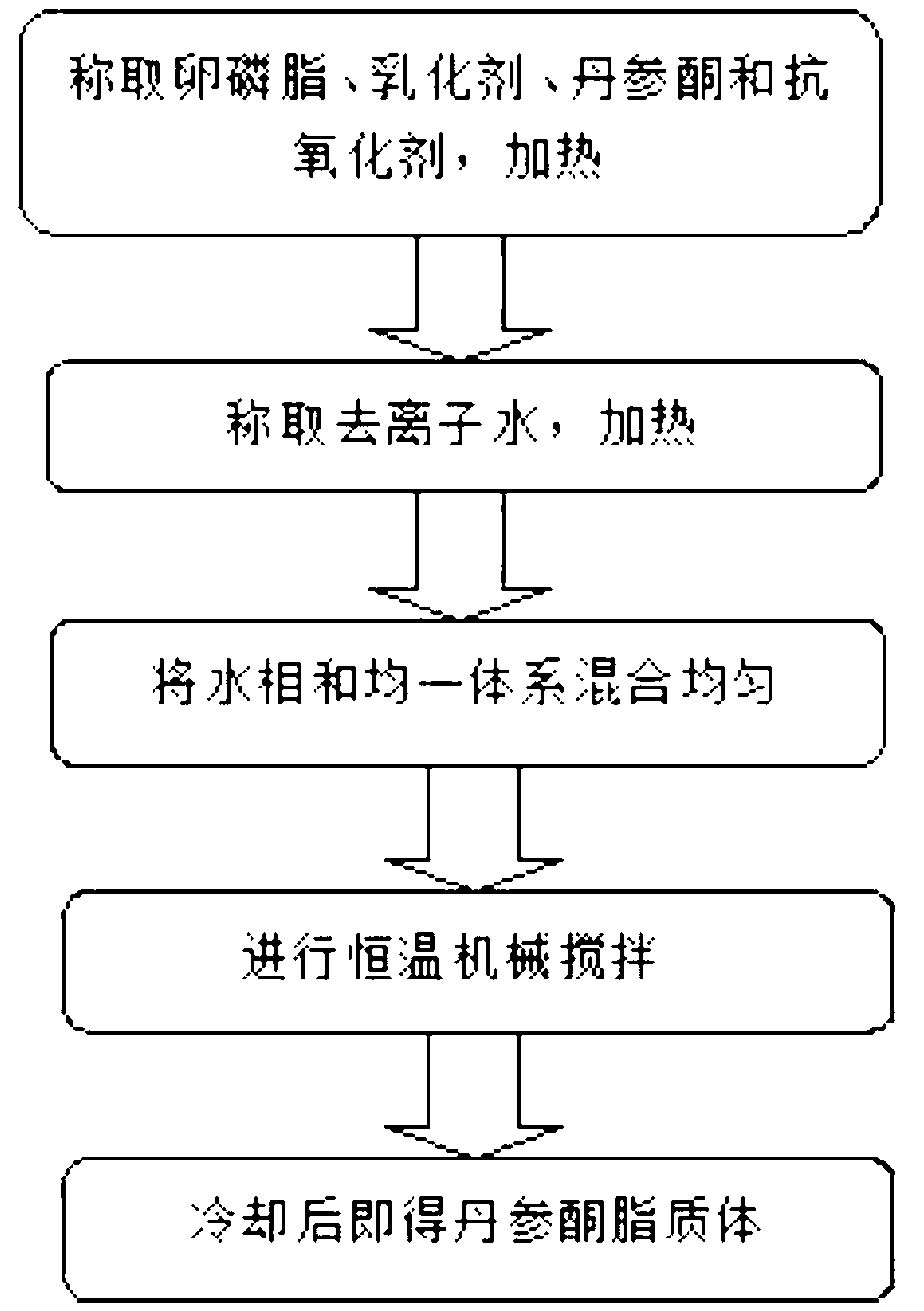
InactiveCN102697664AImprove stabilityGood water solubilityCosmetic preparationsToilet preparationsVitamin E AcetateAntioxidant
The invention discloses tanshinone lipidosome which takes tanshinone as the carrier and has the effects of resisting bacteria, resisting resistance, removing acne and eliminating acne marks. The tanshinone lipidosome is characterized in that the preparation method of the lipidosome is a propylene glycol injection method and the carrier comprises the following components according to weight percent: 0.01 to 5% of tanshinone, 8 to 25% of emulsifier, 2 to 18% of lecithin, and 0.1 to 1% of antioxidant, and the rest is deionized water, wherein the material of emulsifier is a compound taken from atleast two of the following materials: Tween 20, Tween 60, Tween 80, PEG-200, alcohol, propylene glycol and 1,3-butanediol; the material of lecithin is taken from the following compounds: egg yolk lecithin and soya lecithin; and the antioxidant is vitamin E acetate. The lipidosome preparation agent has good stability and can carry high-content tanshinone; and the preparation method is simple and controllable, has good repeatability and can be applied to cosmetic for removing acne and eliminating acne marks.
Owner:SOUTHEAST UNIV
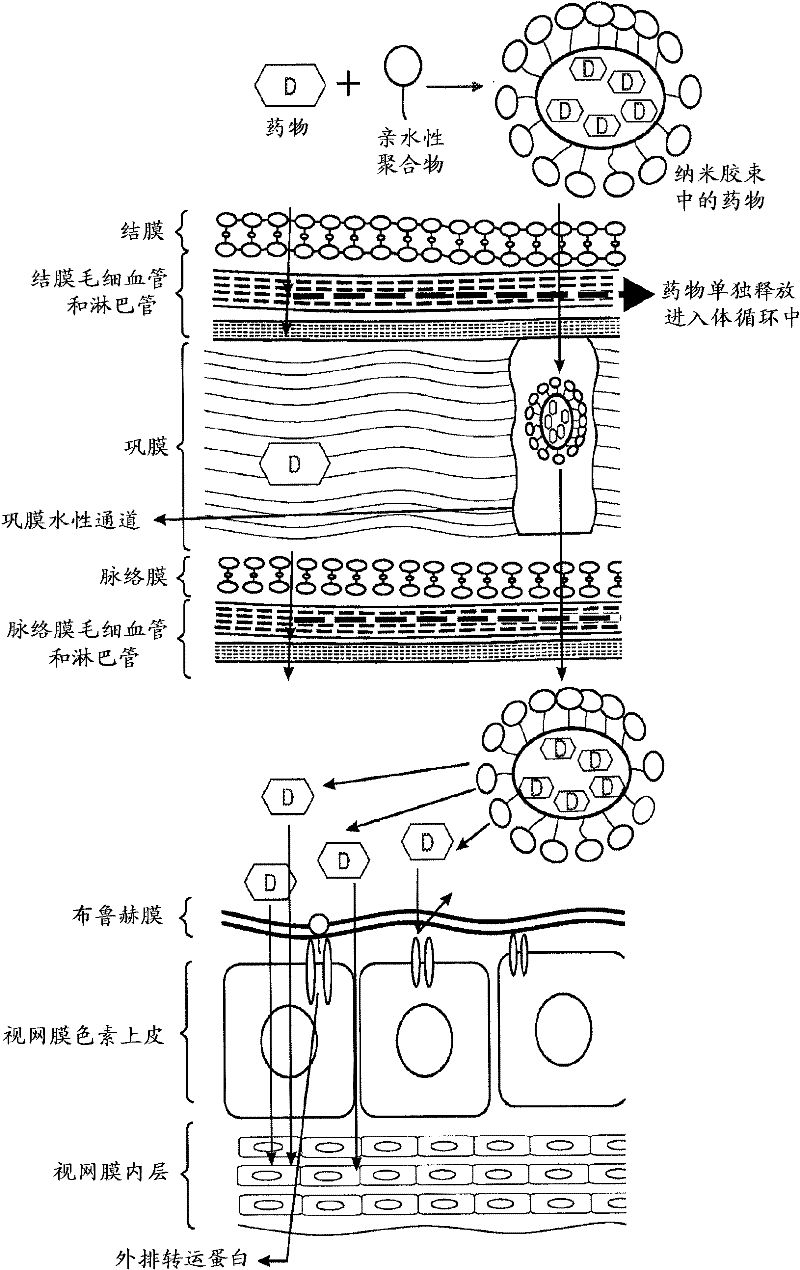
Topical drug delivery systems for ophthalmic use
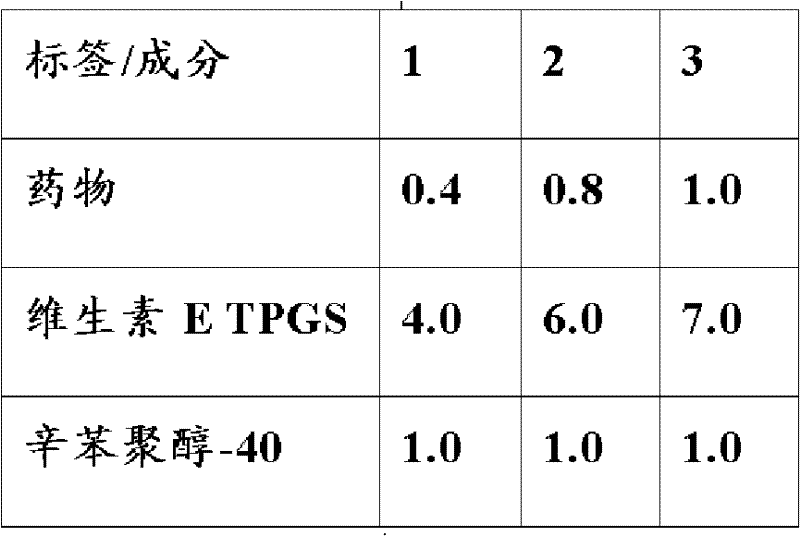
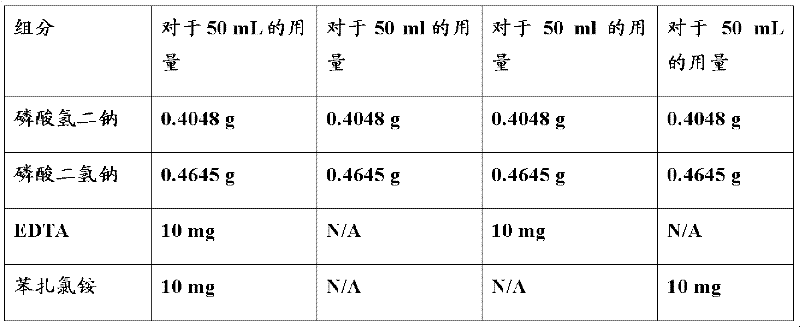
Topical drug delivery systems for ophthalmic use including mixed nanomicellar formulations of water-insoluble drugs and methods of treating diseases affecting the posterior ocular segments are disclosed. In an embodiment, an aqueous ophthalmic solution includes nanomicelles in a physiologically acceptable buffer, having a pH of 5.0 to 8.0, wherein a corticosteroid at a concentration from about 0.01 % w / v to about 1.00 % w / v is solubilized through entrapment in a mixed micellar hydrophobic core with a corona composed of hydrophilic chains extending from the hydrophobic core, wherein the nanomicelles comprise vitamin E TPGS at a concentration ranging from about 3.0 % w / v to about 5.0 % w / v stabilized with octoxynol-40 at a concentration ranging from about 1.0 % w / v to about 3.0 % w / v.
Owner:AURINIA PHARMA
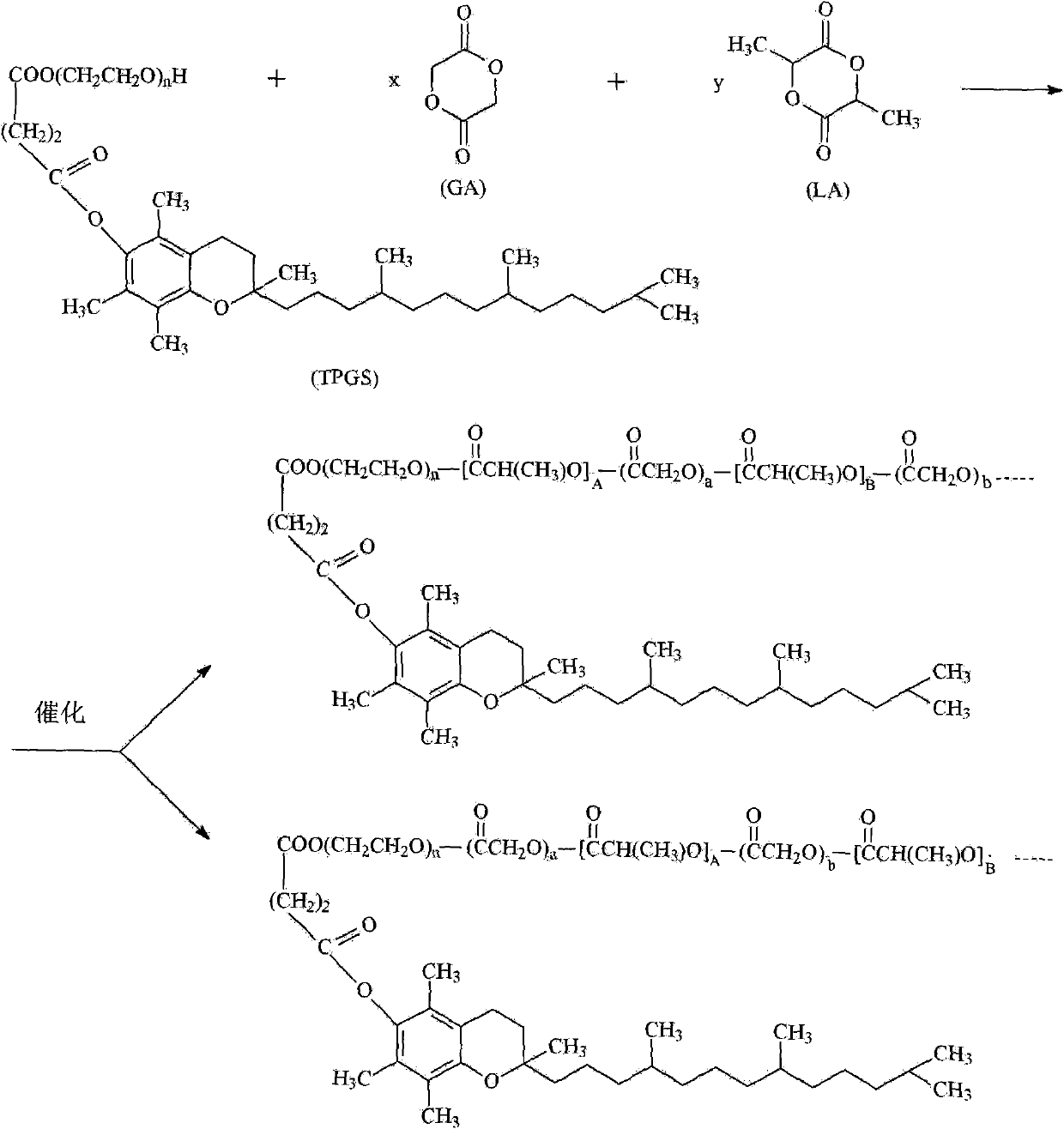
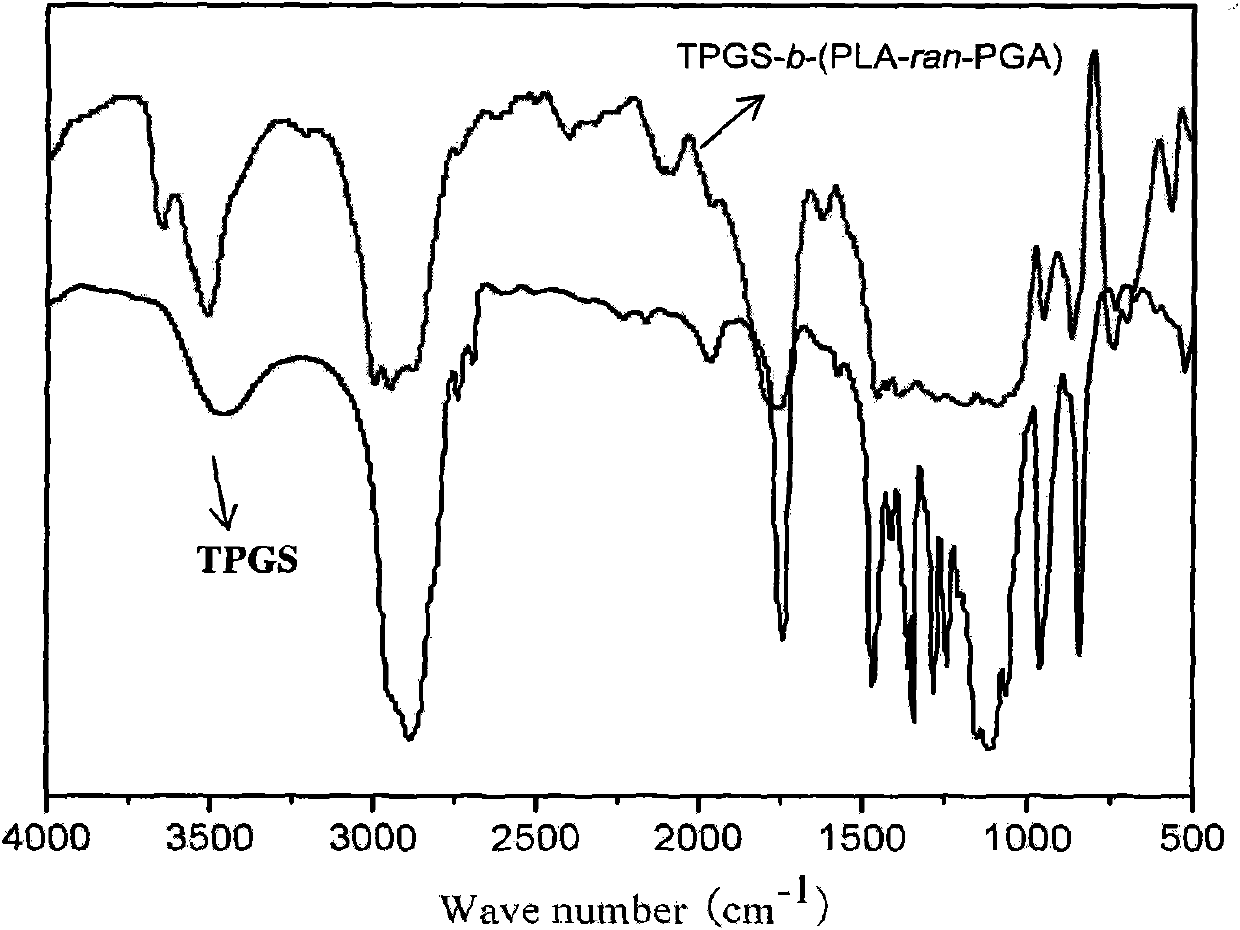
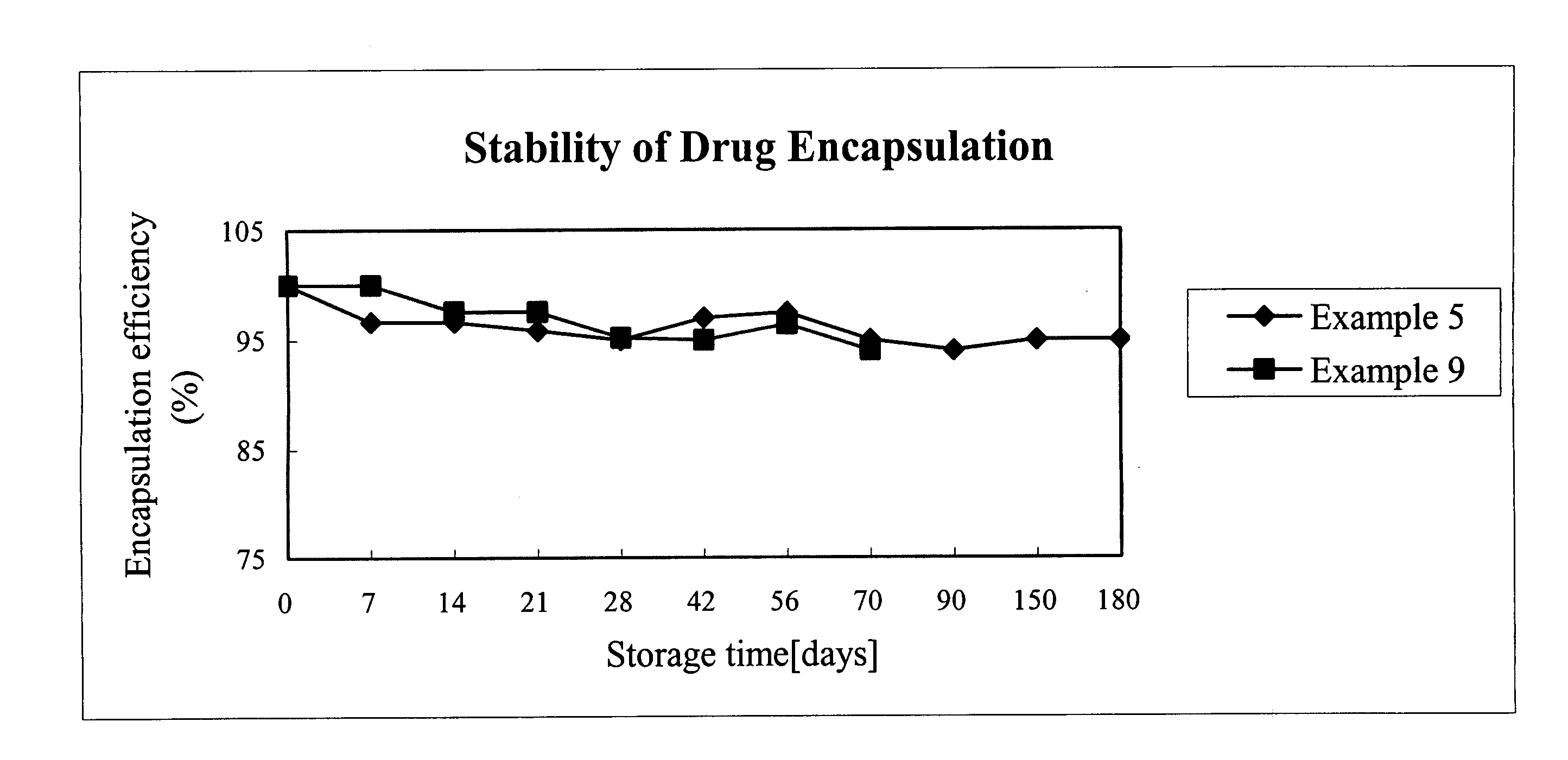
TPGS-b-(PLA-ran-PGA) copolymer and preparation method and application thereof
InactiveCN102030891ASimple methodGood biophaseSuture equipmentsPowder deliveryBiocompatibility TestingOxygen
The invention discloses D-alpha-tocopherol polyethylene glycol 1000 succinate (TPGS)-b-(polylactide (PLA)-ran-polyglycolide (PGA)) copolymer and a preparation method and application thereof. Structural formulas of the copolymer are shown as a formula I and / or a formula II, wherein n is 20 to 140, A+B+... is 2 to 690, and a+b+... is 2 to 840. The preparation method comprises the following steps of: putting 2 to 95 mass percent of lactide monomers, 2 to 95 mass percent of glycolide monomers and 3 to 40 mass percent of D-alpha-tocopherol polyethylene glycol 1000 succinate serving as raw materials into a reactor; adding a catalyst which accounts for 0.1 to 1 percent based on the total mass of the raw materials; and reacting in the absence of oxygen at the temperature of between 140 and 160 DEG C for 12 to 20 hours to obtain the TPGS-b-(PLA-ran-PGA) copolymer. The TPGS-b-(PLA-ran-PGA) copolymer prepared by the invention has high biocompatibility and biodegradability, and is applied to the fields of medicinal preparations and tissue engineering, particularly the field of medicinal preparations for resisting and treating tumor.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Preparation method of solubilizing agent
The invention discloses a preparation method of a solubilizing agent, and belongs to the technical field of solubilizing agents. The method comprises the steps that to-be-purified vitamin E-polyethylene glycol-succinate is dissolved in an organic solvent, heated to be dissolved, washed for 1-5 times with saline water, dried with a drying agent after washing, and filtered after drying, the organicsolvent is steamed out to obtain vitamin E polyethylene glycol succinic acid ester with low content of polyethylene glycol; the organic solvent is selected from ethyl acetate, butyl acetate or methylbenzene; the content of the polyethylene glycol in the to-be-purified vitamin E-polyethylene glycol-succinate is 5-30% by weight, and the content of the vitamin E-succinate is less than 0.5% by weight.The preparation method of the solubilizing agent has the advantages that simple technology and low cost are used to purify the crude vitamin E-polyethylene glycol-succinate, the operation is simple,no special equipment is needed, the polyethylene glycol in the product can be effectively removed and controlled in a certain range, and the vitamin E succinate is reserved.
Owner:辅创科技(宜昌)有限公司
Liposome and preparation method of the same
InactiveUS20080292688A1Improve solubilityImprove long-term stabilityCosmetic preparationsBiocideAlpha-TocopherolLiposome
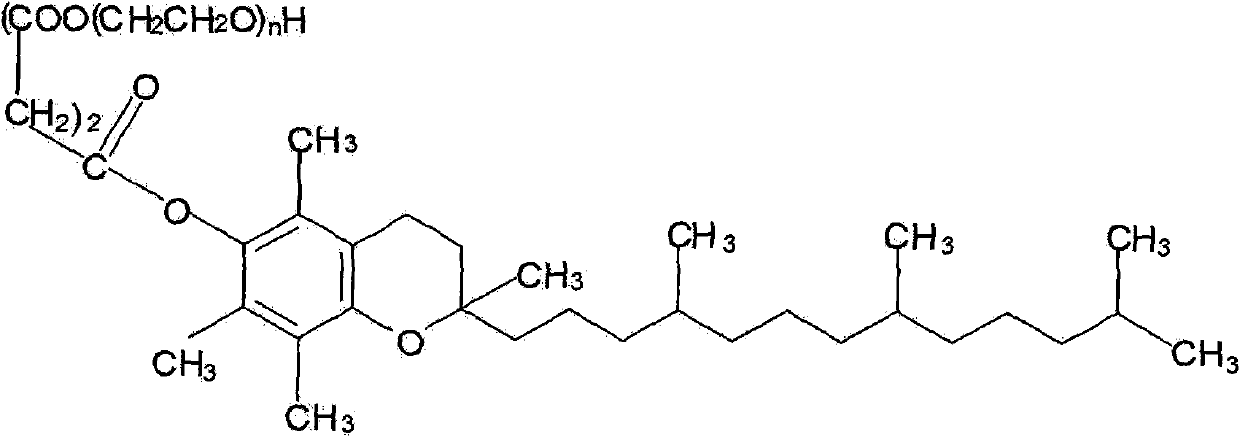
The present invention relates to a composition and a method for preparing a liposome, the liposome including a lipid bilayer and an aqueous core contains a hydrophobic or a hydrophilic drug and a component—Vitamin E derivative (d-α tocopheryl polyethylene glycol 1000 succinate; TPGS). TPGS is able to increase the encapsulation efficiency of drug in liposome as well as to enhance the stability of drug in liposomes. Such liposome is capable to increase the skin permeation of drugs. The preparation method comprises the following steps: (1) adding the drug to a Vitamin E derivative solution to form a mixture; and (2) adding at least one phosphatidyl choline to the mixture, after hydration from either sonication or homogenization.
Owner:IND TECH RES INST
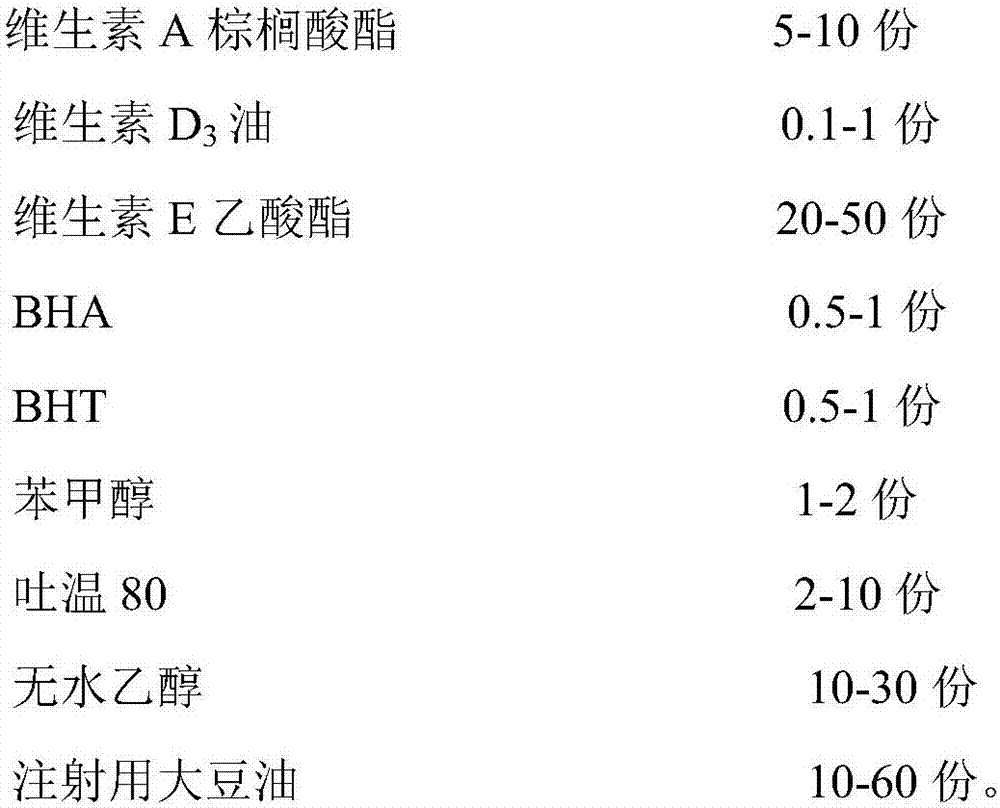
Vitamin ADE microemulsion injecta for veterinary use and preparation method of vitamin ADE microemulsion injecta
InactiveCN107115349APromote absorptionImprove hydrophilicityHydroxy compound active ingredientsAntipyreticVitamin E AcetateSolubility
The invention belongs to the field of veterinary drug preparations, particularly belongs to the technical field of fat-soluble vitamin injection and relates to vitamin ADE microemulsion injecta for veterinary use and a preparation method of the vitamin ADE microemulsion injecta. The vitamin ADE microemulsion injecta for the veterinary use is prepared from the following raw materials in parts by weight: 5 to 10 parts of vitamin A palmitate, 0.1 to 1 part of vitamin D 3 oil, 20 to 50 parts of vitamin E acetate, 0.5 to 1 part of BHA (Butylated Hydroxyanisole), 0.5 to 1 part of BHT (Butylated Hydroxytoluene), 1 to 2 parts of phenylcarbinol, 2 to 10 parts of Tween 80, 10 to 30 parts of absolute ethyl alcohol and 10 to 60 parts of soybean oil for injection. The invention also provides the preparation method of the injecta. According to the preparation method of the vitamin ADE microemulsion injecta, disclosed by the invention, vitamin A, vitamin D and vitamin E are compounded for use; the latest international recommended dose of vitamin is used as the basis of the content of a main drug, grease is selected as a solvent according to the solubility property of the vitamin and is compounded with a certain amount of a surfactant and a cosurfactant, and thus a clear vitamin ADE injecta is obtained; the hydrophilicity and the dispersibility of the product are increased, rapid absorption of an organism is facilitated and the bioavailability is improved.
Owner:宁夏智弘生物科技有限公司
Bazedoxifene acetate composition with excellent property
ActiveCN103845336AGood chemical stabilityFast drug releaseSkeletal disorderPharmaceutical non-active ingredientsSolid solutionTocopherol polyethylene glycol succinate
The invention discloses a bazedoxifene acetate composition with excellent properties. The composition comprises bazedoxifene acetate, polyethylene glycol (PEG) 6000-8000 and vitamin E tocopherol polyethylene glycol succinate (TPGS), wherein a solid solution and / or solid sol is formed by polyethylene glycol (PEG) 6000-8000 and vitamin E TPGS, and bazedoxifene acetate is dispersed into the solid solution and / or solid sol. The composition is high in chemical stability and medicine release speed.
Owner:JIANGSU SEMPOLL PHARMA
Application of vitamin K1 fat emulsion injection
The invention relates to application of a fat emulsion injection using a vitamin K1. Particularly, the invention relates to an emulsion composition comprising the vitamin K1, soybean oil, phospholipid, glycerin and water. The emulsion composition is prepared by processes of preparation of a aqueous phase, preparation of an oil phase, preparation of a coarse emulsion, high-pressure emulsification,hot press sterilization and the like. The invention further relates to a method for preparing the emulsion composition and pharmaceutical application of the emulsion composition. The emulsion injection is used for insufficiency of the vitamin K or coagulation disorder diseases caused by dyssynthesis of coagulation factors II, VII, IX and X, which are generated due to pharmacological intervention interfering the activity of the vitamin K. The emulsion composition disclosed by the invention shows one or various technical effects as shown in the specification.
Owner:西安安健药业有限公司
Stabilized Vitamin D Formulations
The present invention relates to stable solid formulations of vitamin D3 and to processes for preparation of the same. The present invention provides stabilized compositions comprising vitamin D3 at least one lipophilic dispersant, one or more antioxidants, at least one adsorbent and one or more pharmaceutically acceptable excipients and further coated with a barrier coating.
Owner:PSM HEALTHCARE
Cannabinoid emulsion composition and method of manufacture
PendingUS20220241199A1Promote resultsLong period of timeCosmetic preparationsMaterial nanotechnologyVitaminCannabivarin
A method for forming an emulsion containing a cannabinoid includes the steps of first providing a composition comprising water, vitamin E TPGS, a cannabinoid, and a carrier oil, in a high pressure container. The composition is initially opaque. The composition is then heated to a temperature between 50-120 degrees Celsius, the heating increasing the pressure in the high pressure container to greater than 1 ATM. The high pressure container is then cooled, while mixing, until the composition turns clear, and a micelle water emulsion is formed with particles under 100 nm in particle size.
Owner:GORE DOUGLAS
Method for preparing vitamin E nano liposome
InactiveCN101502326BAchieve protectionAchieve sustained releaseOrganic active ingredientsAntinoxious agentsWater basedWater dispersible
The invention provides a preparation method of a vitamin E nano liposome, belonging to the functional health food technology field. The vitamin E is used as core material and lecithin, cholesterol and Tween 80 are used as wall material and vitamin E common liposome is prepared using an ethanol injection method and then subjected to ultra-high pressure homogeneity, thus transparent or semi-transparent vitamin E nano liposome with average diameter of les than 100nm and encapsulation rate of higher than 90% is obtained. The preparation method is suitable for continuous production and solves problem of saled vitamin E in aspects of stability, water dispersibility and oral absorbency and is widely applied on preparation method of functional water based food.
Owner:JIANGNAN UNIV
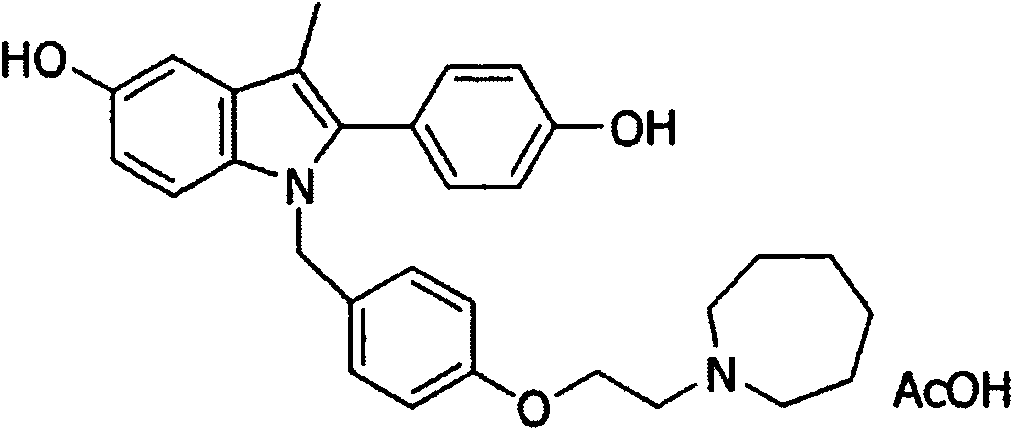
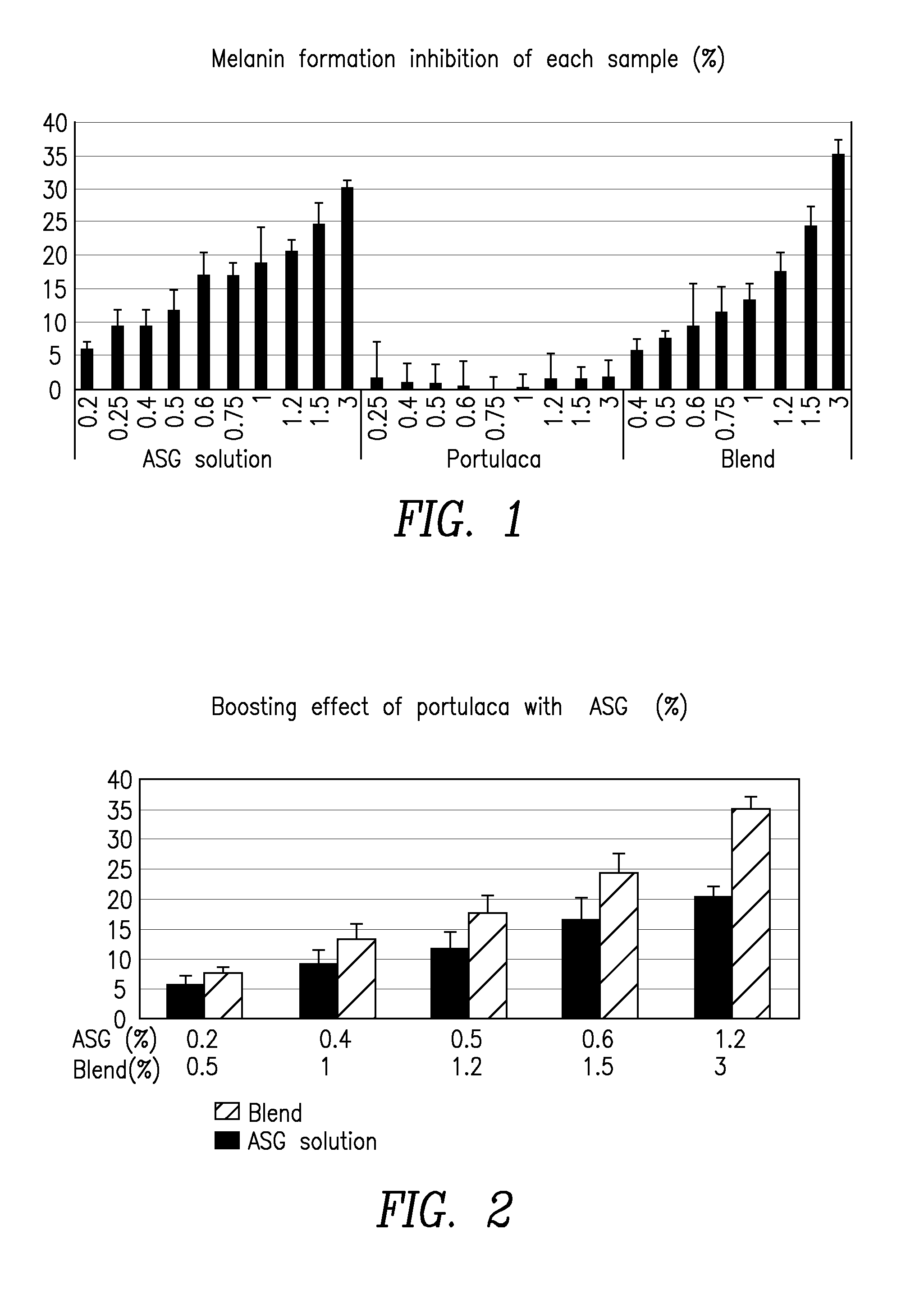
Compositions of vitamin c derivative and portulaca extract
Compositions including Portulaca extract and a vitamin C derivative selected from ascorbyl glucoside (ASG), magnesium ascorbyl phosphate an ethyl ascorbic acid. The compositions can be employed as an inhibitor to melanin formation and used as an additive to a cosmetic or pharmaceutical formulation.
Owner:PRESPERSE CORP
Water-soluble cannabinoids
PendingUS20210186893A1High water concentrationReduce color variationDispersion deliveryHydroxy compound active ingredientsAntioxidantCannabinoid
Provided is a composition comprising: a polysorbate, vitamin E TPGS, a cannabinoid, and an antioxidant. Also provided is a use of the composition for oral consumption and / or topical application and a method of making the composition.
Owner:OROCHEM TECH INC
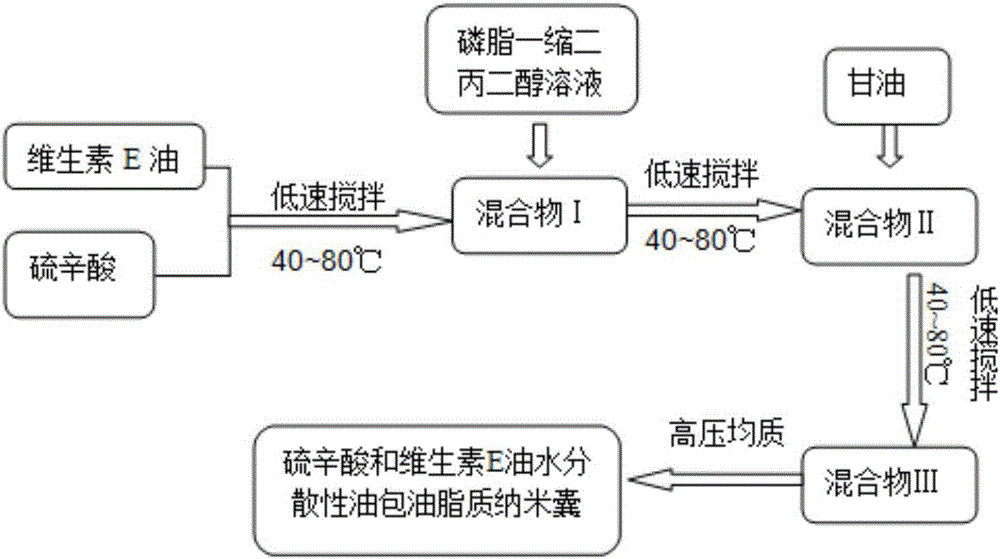
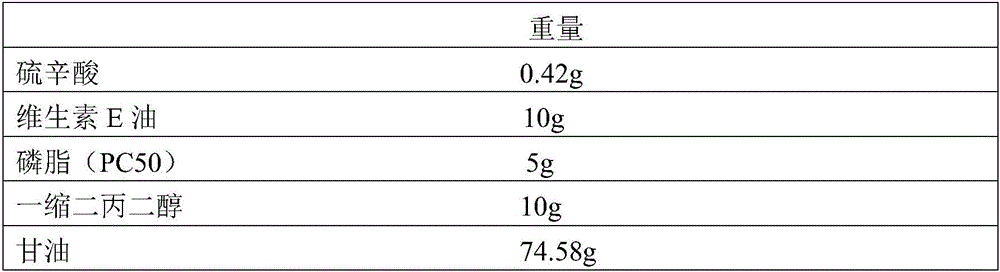
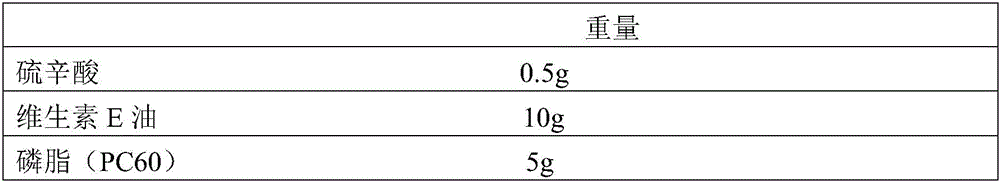
Water-dispersible oil-in-oil lipid nanocapsule and preparation method thereof
InactiveCN106309152AImprove permeabilityGood effectCosmetic preparationsToilet preparationsDispersityWater dispersible
The invention relates to a water-dispersible oil-in-oil lipid nanocapsule containing lipoic acid and vitamin E oil and a preparation method of the water-dispersible oil-in-oil lipid nanocapsule. The preparation method includes: sequentially evenly mixing, by weight percentage, 0.1-5% of the lipoic acid, 0.1-20% of vitamin E oil, 1-10% of phospholipid, 2-20% of dipropylene glycol, 50-80% of glycerin and the like at 40-80 DEG C, and performing high-pressure homogenizing treatment to obtain the transparent or semitransparent water-dispersible oil-in-oil lipid nanocapsule containing the lipoic acid and the vitamin E oil, wherein the particle size of the water-dispersible oil-in-oil lipid nanocapsule is 250 nanometers. The water-dispersible oil-in-oil lipid nanocapsule is simple to prepare, good in water dispersity, good in bioavailability, good in repeatability, and the like.
Owner:SOUTHEAST UNIV
Water-soluble coagulant drug vitamin k2 solid-state complex and preparation method thereof
The invention discloses a water-soluble coagulant drug vitamin k2 solid-state complex and a preparation method thereof, and belongs to the technical field of medicines. The product is a solid-state complex of mono-[6-(diethylenetriamine)-6-deoxy]-[beta]-cyclodextrin and vitamin K2 at a mixing ratio of 2 to 1. The mono-[6-(diethylenetriamine)-6-deoxy]-[beta]-cyclodextrin is obtained by adding mono-(6-o-6-p-toluenesulfonyl)-[beta]-cyclodextrin to diethylenetriamine, conducting a reaction in a mode of dissolving and increasing temperature in a nitrogen atmosphere, adding acetone after most diethylenetriamine is distilled so as to obtain a crude product, dissolving the crude product in a water and separating the dissolved product so as to obtain the mono-[6-(diethylenetriamine)-6-deoxy]-[beta]-cyclodextrin. A vitamin K2 acetone solution is added to a mono-[6-(diethylenetriamine)-6-deoxy]-[beta]-cyclodextrin water solution, and it conducts stirring at 35-40 DEG C in a sealed mode for 4.5-5.5 days so as to remove acetone and vitamin K2 failing to participate in inclusion. The obtained product (the solid-state complex) is low in toxicity; and the solubility of the vitamin K2, through inclusion, in water is increased by 110 times, and the product, as a vitamin K2 substitute, is applicable to the clinical field.
Owner:YUNNAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com