Transdermal delivery of cannabidiol with other active moieties including cannabinoids
a technology of cannabinoids and cannabinoids, which is applied in the direction of organic active ingredients, plant/algae/fungi/lichens ingredients, pharmaceutical non-active ingredients, etc., can solve the problems of reducing the bioavailability of cbd, limiting the rate of mass transfer of cbd through the skin, and the adhesive used in such known devices has typically limited the rate of mass transfer of cbd to the skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
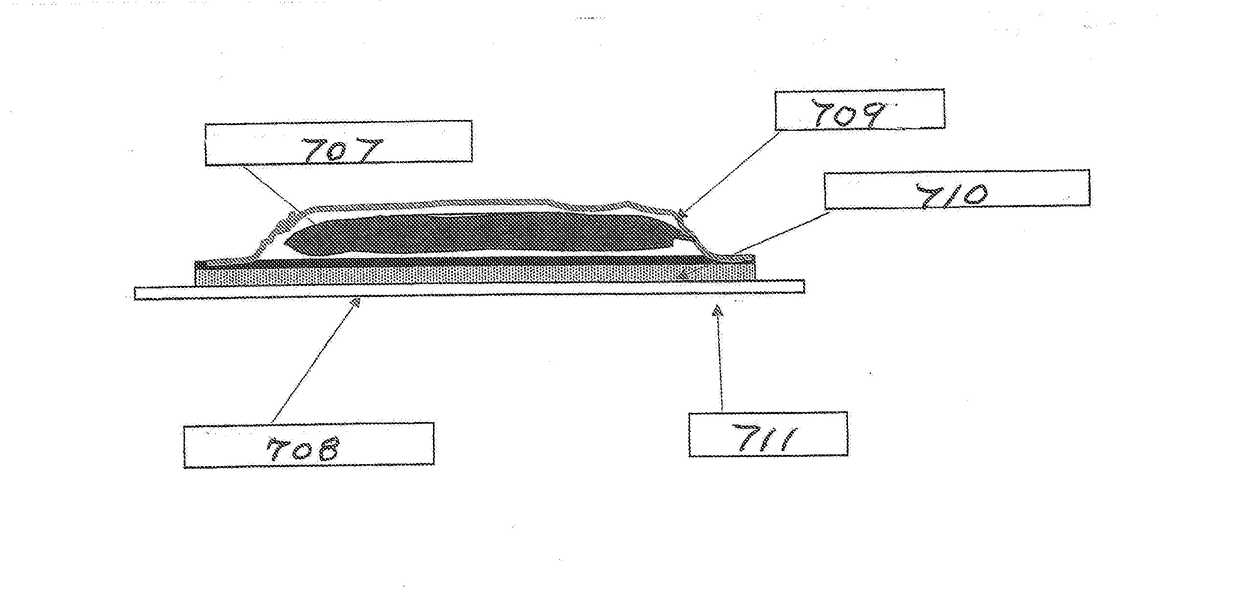
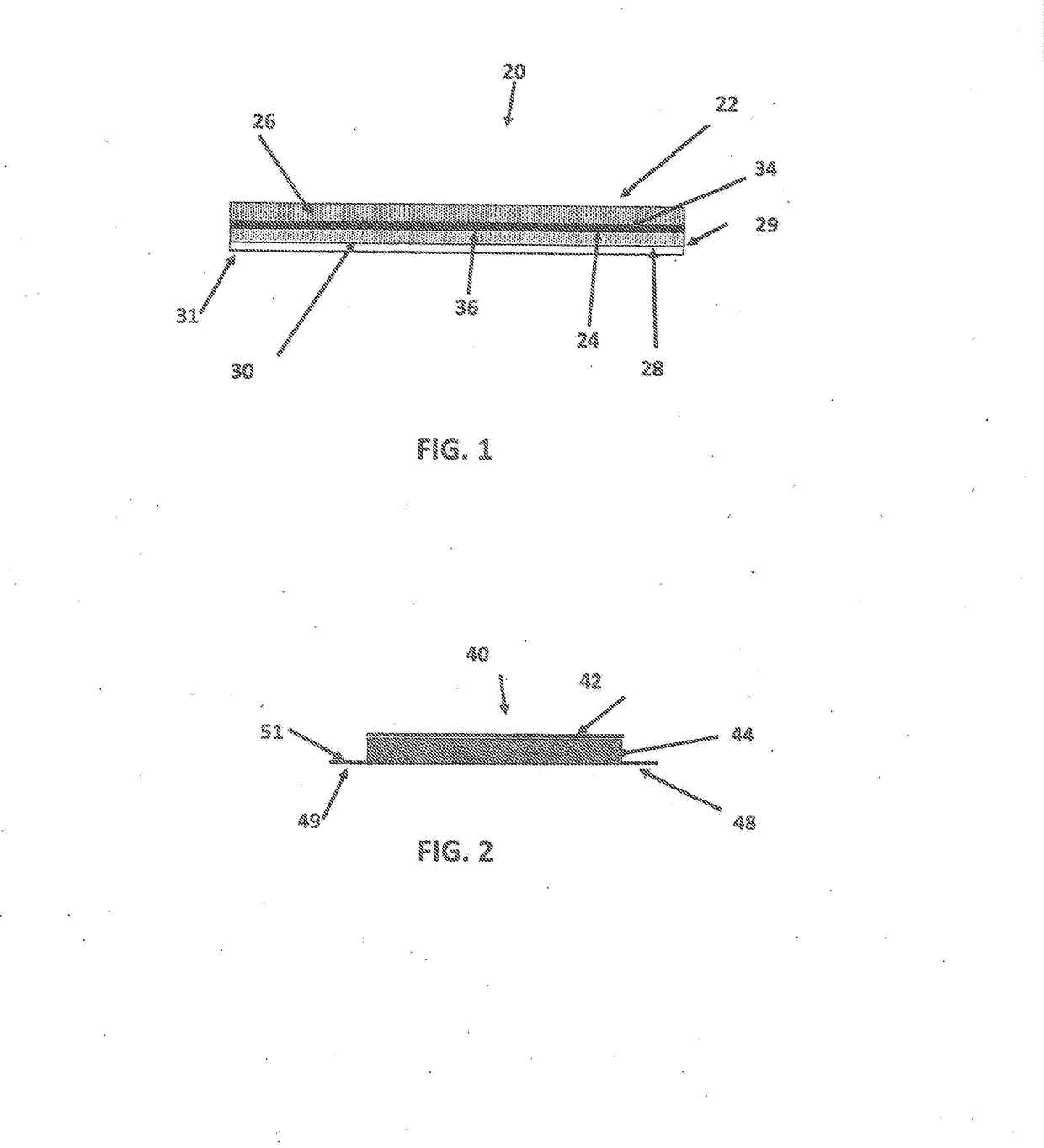
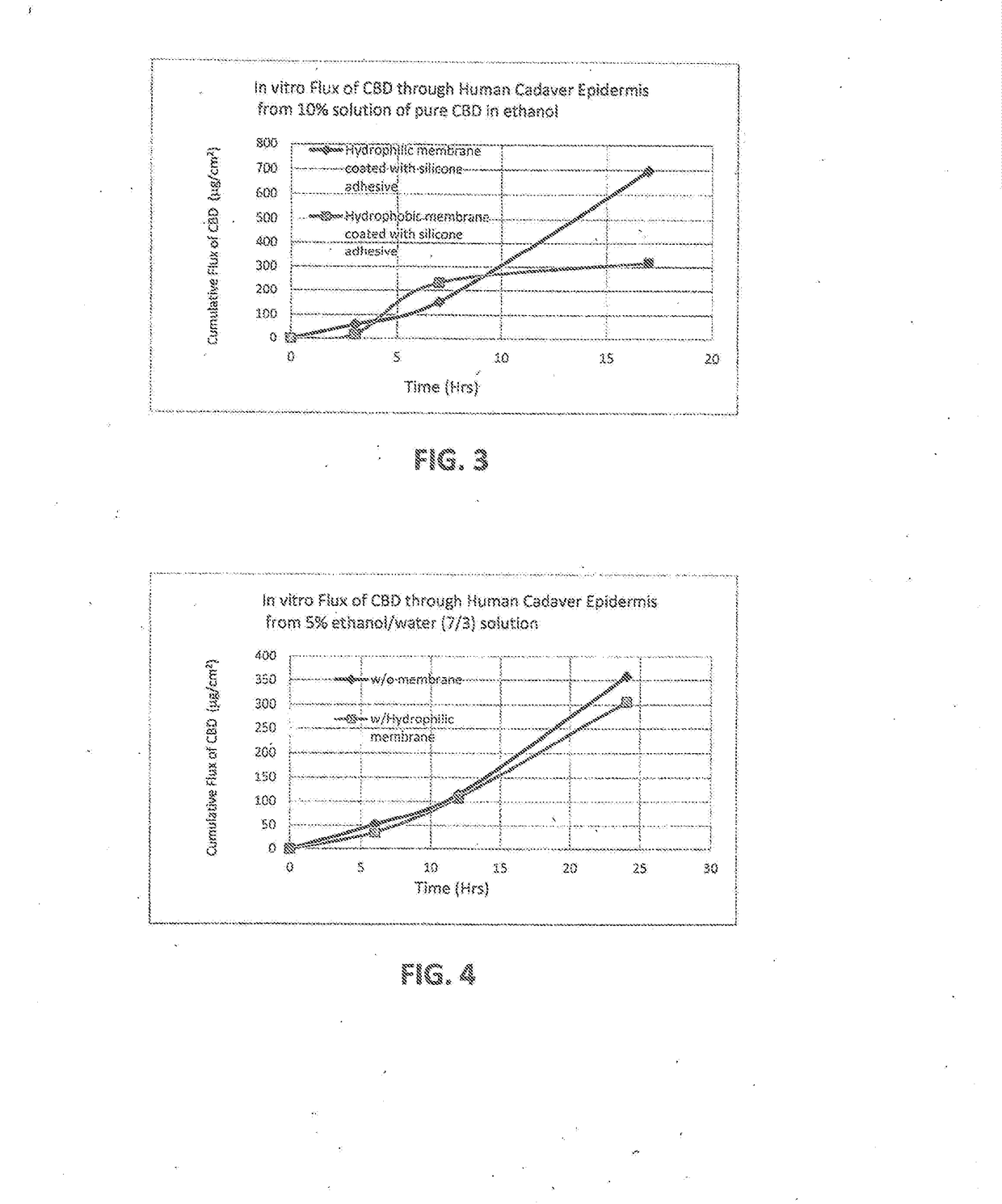
[0112]The effects of using a hydrophobic, porous membrane versus a hydrophilic, porous membrane (such as membrane 24) in reservoir transdermal device 20 are studied in this example. A mixture is formed by dissolving CBD powder in ethanol to yield a solution comprising 10 percent by weight CBD and 90 percent by weight ethanol. The backing 22 is an occlusive backing film (PE / PET from 3M). Hydroxy ethyl cellulose is added to the solution to yield a thixotropic preparation 27. A volume of 300 μL of the preparation is placed in the reservoir 26.
[0113]The skin adhesive 30 is a BIO PSA 7-4301 adhesive coated to a thickness of 15 g / m2 on side 36 of membrane 24. An overlay adhesive patch is placed over the reservoir which forms an island in the middle. The overlay assures adhesion of the reservoir to the skin. The device 20 is contacted with a human cadaver epidermis, and diffusion through the epidermis is measured using the Franz Diffusion Cell method.
[0114]In a first run, membrane 24 is a ...
example 2
[0115]The reservoir device 20 of Example 1 is used, but instead of using ethanol alone as the liquid carrier, a mixture of ethanol and water is used wherein the ethanol comprises 70 percent by weight of the ethanol / water mixture, and water comprises 30 percent by weight of the ethanol / water mixture. The amount of CBD by weight of the combination of CBD and ethanol / water is 5 percent, and the amount of ethanol / water is 91.5 percent. The gelling agent is hydroxyethylcellulose, which is present in an amount of about 3.5% by weight of the preparation 27. A first run is conducted in which the membrane is placed comprises CBD is present
[0116]The device 20 is contacted with a human cadaver epidermis, and diffusion through the epidermis is measured using the Franz Diffusion Cell method. In a first run, the hydrophilic, porous membrane 24 of Example 1 is used. In a second run, the membrane 24 is omitted so that CBD diffuses directly through the skin adhesive 30. The cumulative flux versus ti...
example 3
[0117]This example is conducted using three reservoir transdermal devices such as those of Example 1, with each having the hydrophilic, porous membrane described therein. In each case, the preparation includes a mixture of CBD and a liquid carrier with ten (10) percent CBD by weight of the CBD / liquid carrier mixture. Three runs are conducted, each with a device that includes a different liquid carrier in its preparation 27: 1, 2 propylene glycol, PEG-300, and oleic acid. Diffusion through a human cadaver epidermis is measured using the Franz Diffusion Method.
[0118]The cumulative flux versus time is plotted in FIG. 5. As the figure indicates, throughout the period, oleic acid provides a significantly better rate of transfer of CBD through the skin than either 1,2 propylene glycol or PEG-300.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| mean flow pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com