Water-soluble coagulant drug vitamin k2 solid-state complex and preparation method thereof
A vitamin and procoagulant technology, applied in the field of medicine, to achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
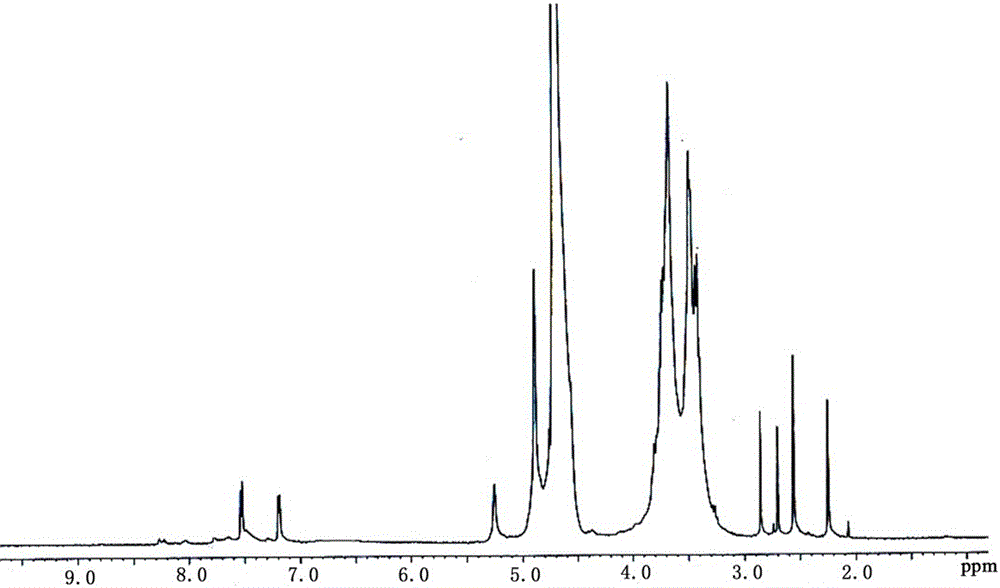
[0036] Mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin and vitamin K involved 2 The solid complexes were synthesized as follows:
[0037] 1) Synthesis of mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin 2 Mono-[6-(diethylenetriamine)-6-deoxy]-β-cyclodextrin For the detailed synthetic route, see Figure 14 (Synthesized according to literature, I.Tabushi, N.Shimizu, T.Sugimoto, M.Shiozuka, K.Yamamura, J.Am.Chem.Soc.1977,99,7100; I.Tabushi, N.Shimizu, Jpn.Kodai Tokkyo Koho 78 102 985, 1978 [Chem. Abstr., 1979, 90, 39196b]).
[0038] Into a 100mL dry round-bottomed flask, add 40mL of diethylenetriamine purified by distillation, add 5.0g of mono-(6-oxo-6-p-toluenesulfonyl)-β-cyclodextrin while stirring, protect with nitrogen, and dissolve Raise the temperature to about 76°C and react for 7 hours, then distill off most of the diethylenetriamine under reduced pressure, add the remaining liquid to acetone, collect the precipitate by suction filtration, repeat the operation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com