Application of vitamin E TPGS (d-alpha tocopheryl polyethylene glycol 1000 succinate) in preparing porous drug carrier particles
A technology of vitamins and drugs, applied in the field of porous drug carrier particles, can solve problems such as multidrug resistance, reduce sensitivity to chemotherapy, and easily lead to hypersensitivity reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
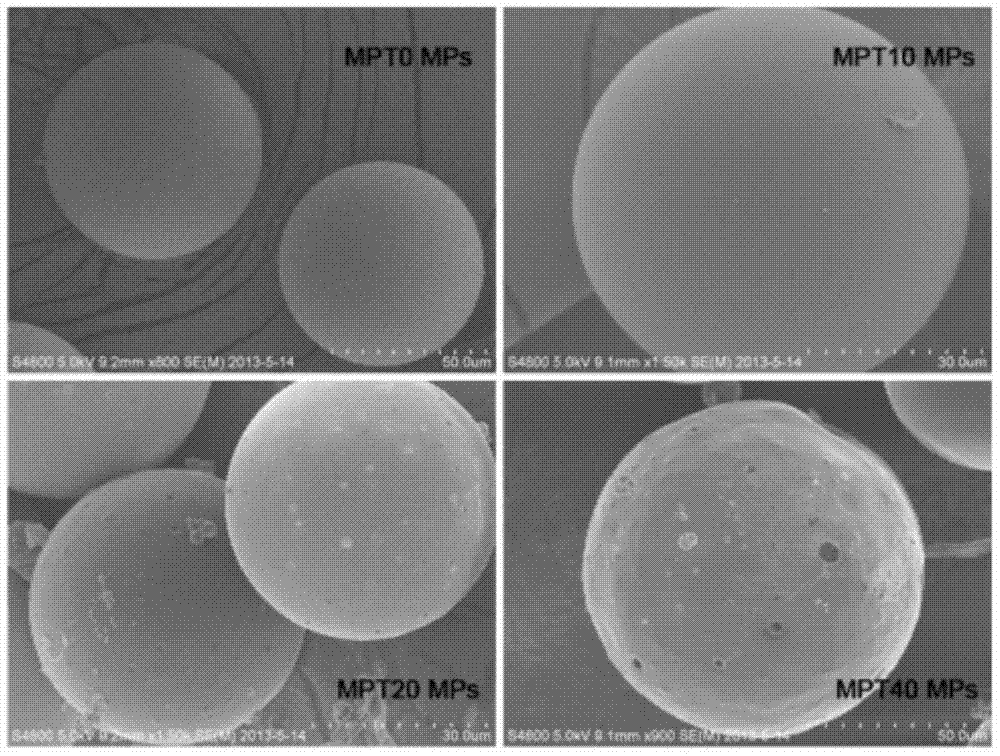
[0070] Embodiment 1, preparation of PLGA microspheres loaded with docetaxel and PLGA / TPGS blended microspheres
[0071] Three kinds of microspheres loaded with docetaxel were prepared by solvent evaporation method. The preparation of MPT0 microspheres (PLGA microspheres) was as follows: 90 mg of PLGA / TPGS mixture and 10 mg of docetaxel were accurately weighed with a precision electronic balance, added to 8 mL of dichloromethane (DCM), and left at room temperature until fully dissolved. Measure 100mL of 0.03% TPGS solution with a graduated cylinder, put it into a 250mL beaker, and add the mixed solution of the first step into it for emulsification under the condition of stirring at a rotating speed of 400rpm. After stirring overnight, the organic solvent was removed and PLGA microspheres were formed. Centrifuge at 5000 rpm for 5 min and wash three times with deionized water to remove attached TPGS and docetaxel. The resulting precipitate was resuspended in 3 mL of deionized w...
Embodiment 2
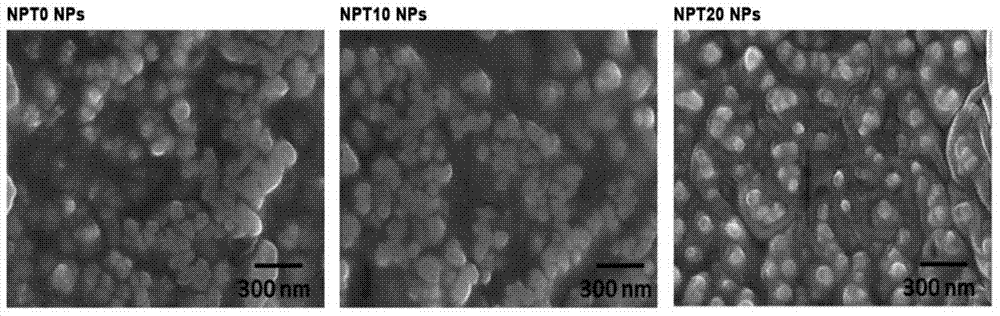
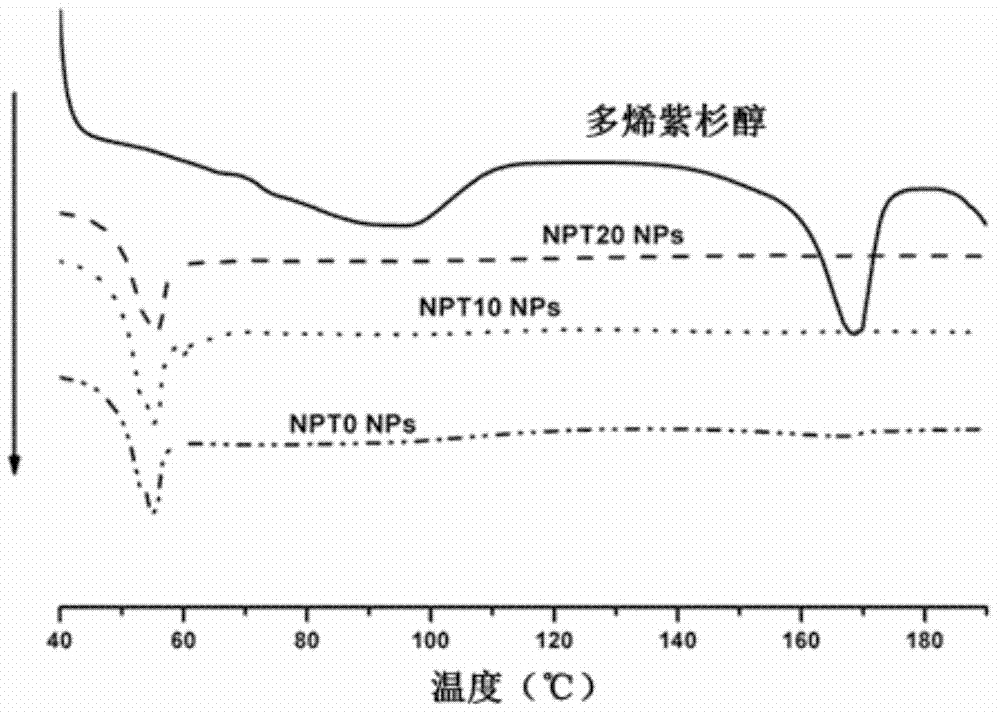
[0074] Example 2, preparation and performance of PLGA nanoparticles loaded with docetaxel (or blank) and PLGA / TPGS blended nanoparticles
[0075] Nanoparticle preparation
[0076] According to the preparation conditions listed in Table 1, PLGA nanoparticles (NPT0) and PLGA / TPGS blended nanoparticles (NPT10 and NPT20) loaded with docetaxel were prepared with reference to the method of Example 1, and the solvent acetone was replaced by dichloro Methane, and the stirring speed was 800rpm for 2h, then reduced to 600rpm and stirred overnight, and the centrifugation condition used for harvesting nanoparticles was 20,000rpm, centrifuged at 4°C for 15min. In addition, blank PLGA nanoparticles and PLGA / TPGS blend nanoparticles were prepared by not adding docetaxel.
[0077] Characterization of Nanoparticles
[0078] Take the NPT0, NPT10 and NPT20 nanoparticle suspensions before freeze-drying, use dynamic light scattering to analyze the particle size and particle size distribution, an...
Embodiment 3
[0103] Example 3, preparation and characteristics of PLGA nanoparticles loaded with coumarin and PLGA / TPGS blended nanoparticles
[0104] Nanoparticle preparation
[0105] According to the preparation conditions listed in Table 1, with reference to the method of Example 2, the PLGA / TPGS blended nanoparticles loaded with coumarin-6 were prepared, except that docetaxel was replaced by coumarin-6, and the obtained nanoparticles Denote as NPT0C, NPT10C and NPT20C, wherein the percentage of TPGS in the PLGA / TPGS mixture when the numerical table is prepared.
[0106] Characterization analysis
[0107] Take the NPT0C, NPT10C and NPT20C nanoparticle suspensions before freeze-drying, use dynamic light scattering to analyze particle size and particle size distribution, and use laser Doppler velocimetry to measure the zeta potential of nanoparticles. Three replicate measurements were performed. Results are expressed as mean ± standard deviation (SD). The results are shown in Table 4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com