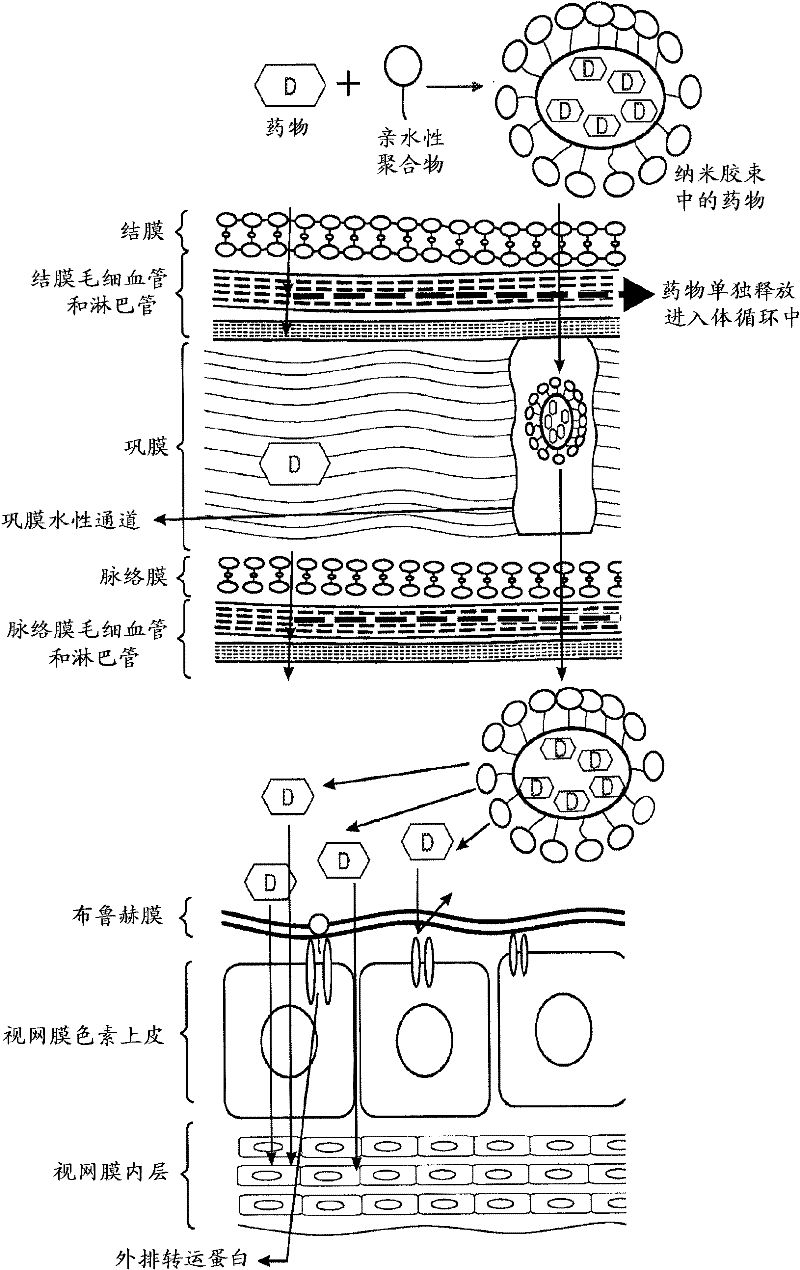
Topical drug delivery systems for ophthalmic use
A water eye and eye drop technology, which is applied in the direction of drug delivery, drug combination, powder delivery, etc., can solve the problems of increasing tissue damage and infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of mixed nanomicelle formulations
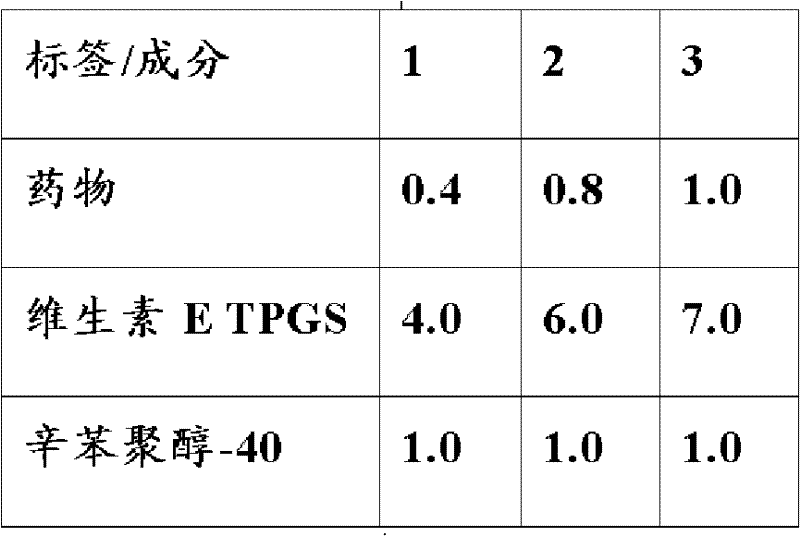
[0086] The mixed nanomicelle formulations of the present invention with drug concentrations of 0.02 wt%, 0.2 wt%, 0.4 wt%, 0.5 wt% and 1.0 wt% were prepared as follows. Base 2X drug formulations were prepared in the proportions shown in Table 1. In one protocol, for example, the required calcineurin inhibitor and vitamin E TPGS are calculated for about 50 mL, weighed, and then mixed in about 5 mL of 95% ethanol until a clear solution is obtained. The ethanol solution was evaporated under vacuum to obtain a thin film near solid. About 25 mL of deionized water and octoxynol-40 were mixed, and the solution was added to the film-like near-solid substance, and ultrasonicated for about 20 minutes to ensure complete formation of mixed micelles. Store the prepared 2X drug formulation at room temperature. Alternatively, the amount of drug, vitamin E TPGS, and octoxynol-40 required for 50 mL was calculated, weighed, then mixed in...
Embodiment 2
[0108] After surface application of 0.2wt% / volume voclosporin nanomicelle formulation (LX214) 14 Ocular distribution and pharmacokinetics of C-voclosporin
[0109] NZW rabbits (30 females / 8 males) were used in single dose (SD) and 7-day repeat dose (RD) studies (Table 5A). DB rabbits (16 females) were used in the single dose study (Table 5B). Animals received no treatment (control) or single dose or daily ocular topical application for 7 days (35 μL of 0.2% 14 C-LX214 solution). At indicated time points, blood and ocular tissue radioactivity levels were assessed by burning followed by liquid scintillation counting. Blood voclosporin concentrations were also measured using a validated liquid chromatography method coupled to air pressure ionization mass spectrometry (LC-API / MS / MS).
[0110] Table 5A
[0111]
[0112]
[0113] a Topical formulation containing 0.2% voclosporin. A target dose of ~3 μCi / 35 μL and 70 ng of voclosporin
[0114] b was used as the pre-admin...
Embodiment 3
[0137] Preparation of Dexamethasone Mixed Nanomicelle Preparation
[0138] The aqueous solubility of corticosteroids such as dexamethasone is about 0.159 mg / mL. In this example, the solubility of the drug dexamethasone was increased about 6.2 times (1 mg / mL). In one embodiment, to prepare a composition having a drug (dexamethasone) concentration of 0.1 wt%, the following protocol was used. Pharmaceutical base formulations were prepared according to the ratios shown in Table 9. In this protocol, the dexamethasone, vitamin E TPGS, and octoxynol-40 required for a final 50 mL formulation were calculated, weighed, and then mixed in approximately 6 mL of 95% ethanol until a clear solution was obtained. The ethanol solution was evaporated under vacuum to give a thin film near solid. 25 mL of deionized water was added to the film-like near-solid substance, and ultrasonic treatment was performed for about 20 minutes to ensure complete formation of mixed micelles. The prepared 2X fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com