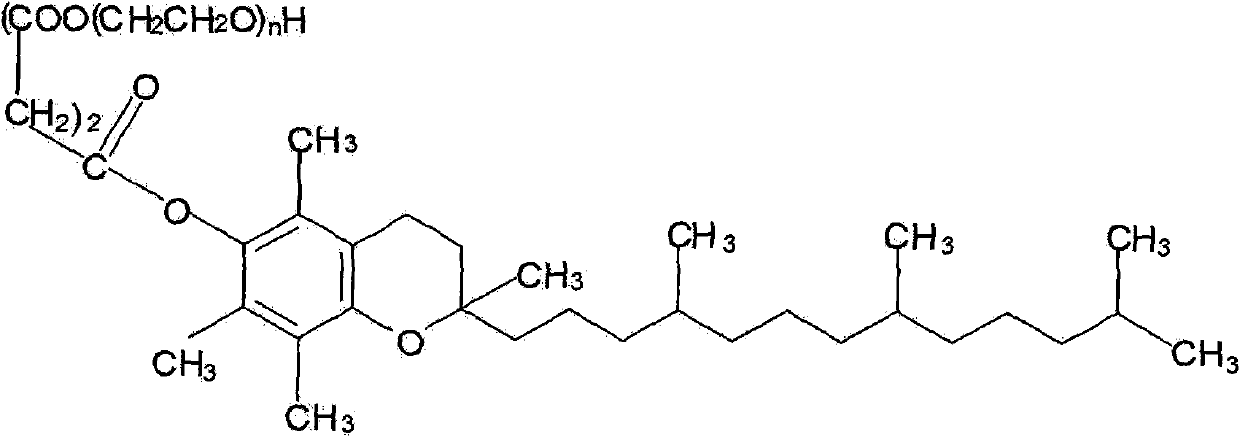
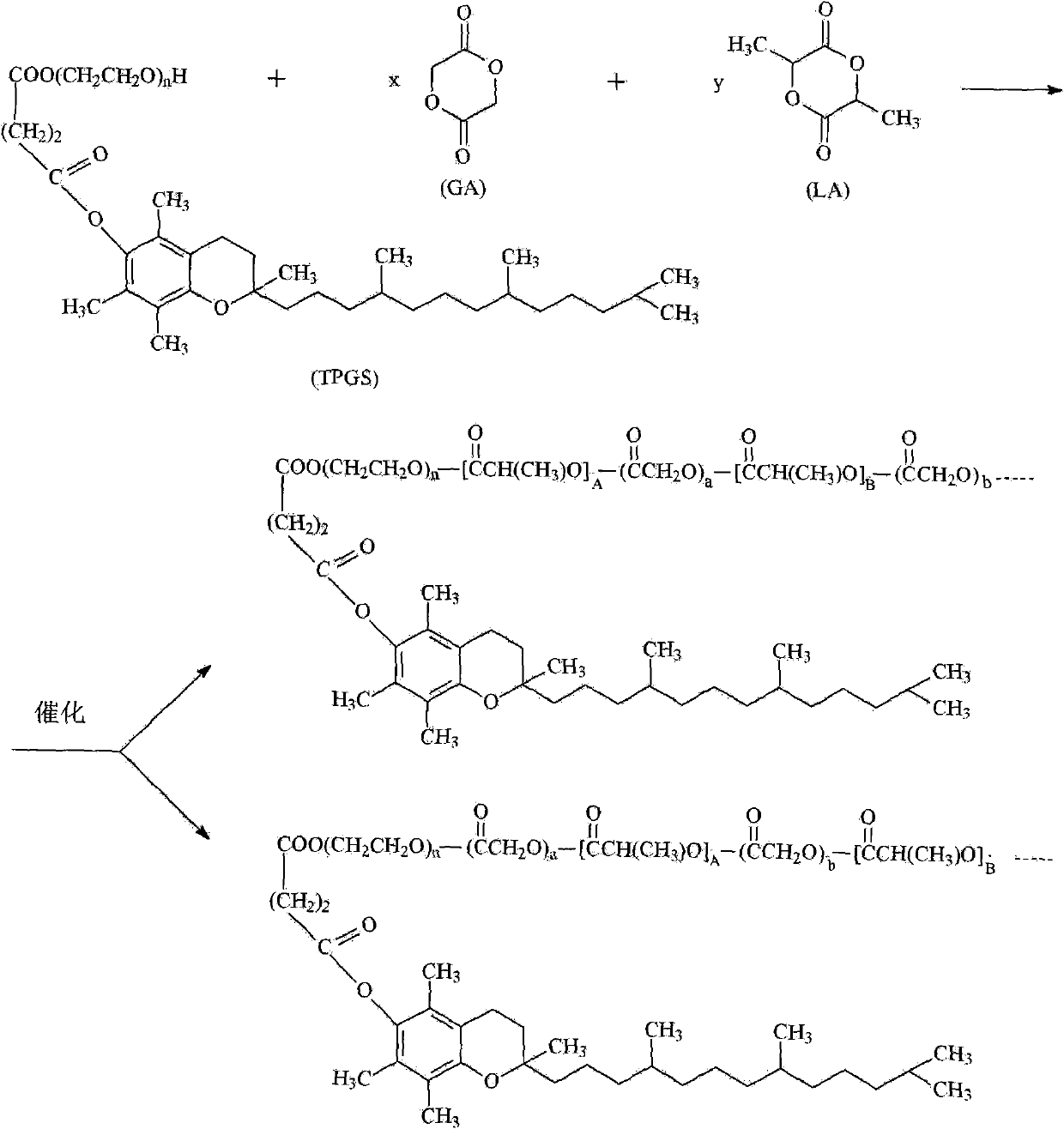
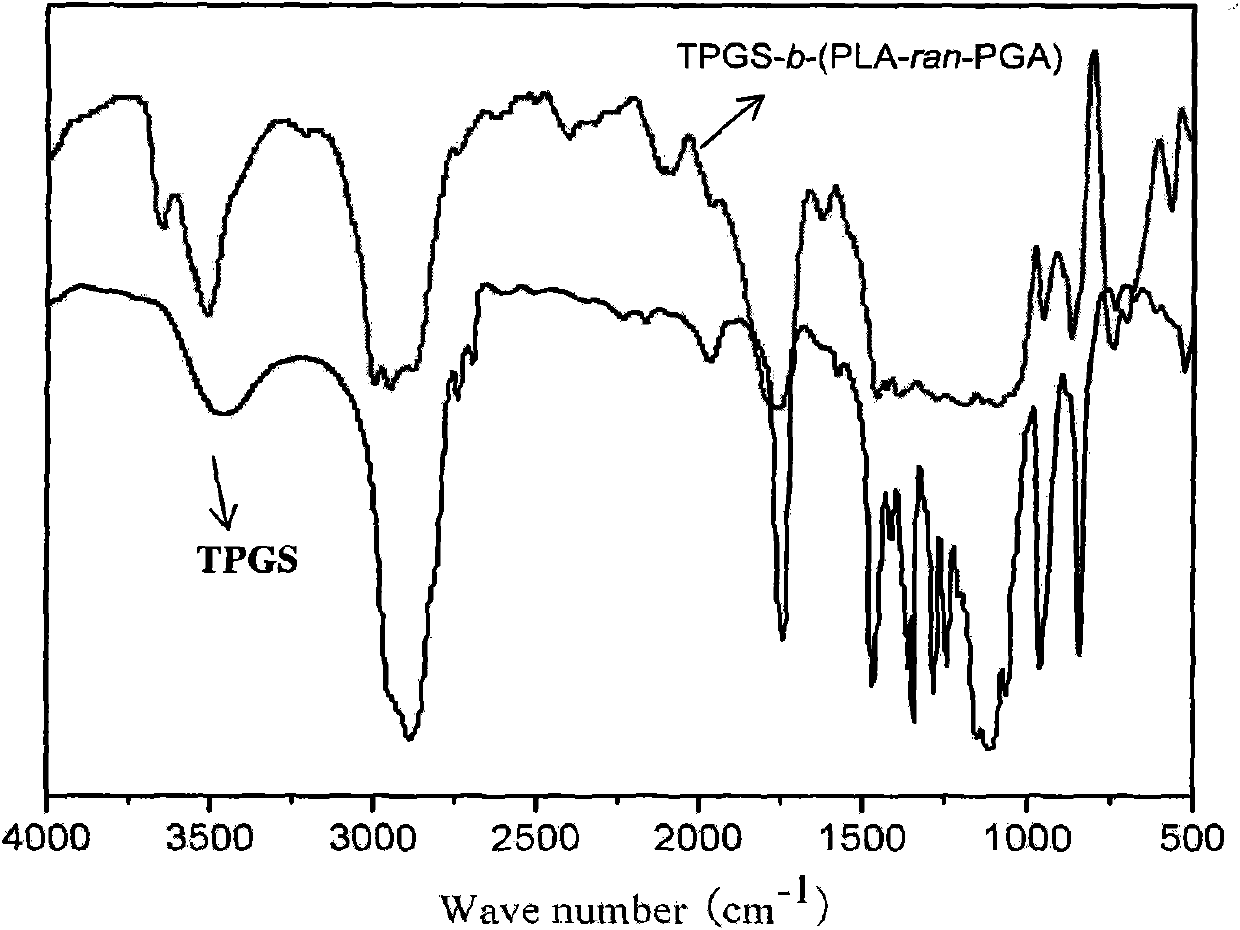
TPGS-b-(PLA-ran-PGA) copolymer and preparation method and application thereof
A technology of pla-ran-pga and copolymer, which is applied in the fields of medical science, powder transportation, bulk transportation, etc., can solve the problem of lack of research, development and application of new pharmaceutical excipients, and achieve the effect of simple method and good biological phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, preparation TPGS-b-(PLA-ran-PGA) copolymer
[0038] Accurately weigh 7.5g (monomer total mass 75%) racemic lactide monomer (D, L-Lactide), 1.5g (monomer total mass 15%) glycolide monomer (Glycolide), 1g ( Monomer total mass 10%) Vitamin E TPGS (n=32, molecular weight 1513) is raw material, gets the catalyst stannous octoate of monomer gross mass 0.1% and adds in the polymer tube, carries out vacuum degassing and nitrogen replacement process, repeats Three times (to ensure the anaerobic condition of the equipment), the polymerization tube was sealed, and the ring-opening polymerization reaction was carried out under heating in an oil bath at 145°C. After 16 hours of polymerization, the product was cooled and dissolved in methylene chloride, and excess methanol was added to precipitate the copolymer. Methanol unreacted monomer and TPGS were removed by filtration. The resulting precipitate was vacuum-dried at 45° C. for 48 hours to obtain the polymer produc...
Embodiment 2
[0044] Embodiment 2, preparation TPGS-b-(PLA-ran-PGA) copolymer
[0045] Take 95% L-lactide monomer, 2% glycolide monomer and 3% polyethylene glycol 1000 vitamin E succinate (TPGS) (n=32, molecular weight 1513) as raw materials according to the mass percentage , put into the polymerization tube, add tetramethyldibutylguanidine acetate accounting for 0.5% of the total mass of the raw material, vacuumize, fill with nitrogen, repeat three times (to ensure the anaerobic condition of the equipment), the polymerization tube is sealed, at 145 ℃ heating, and reacted for 16 hours to obtain TPGS-b-(PLA-ran-PGA) copolymers (I) and (II) with a number average molecular weight of 46,000 (calculation method is the same as in Example 1).
[0046] The TPGS-b-(PLA-ran-PGA) copolymer prepared above, corresponding to (I) and (II), n is about 32, and the lactide segment (A+B+...) value is about 607, glycolide A copolymer with a segment (a+b+...) value of about 16.
Embodiment 3
[0047] Embodiment 3, preparation TPGS-b-(PLA-ran-PGA) copolymer
[0048] Take 58% racemic lactide monomer, 2% glycolide monomer and 40% polyethylene glycol 1000 vitamin E succinate (n=32, molecular weight 1513) as raw materials by mass percentage Put into the polymerization tube, add zinc powder accounting for 1% of the total mass of the raw materials, vacuumize, fill with nitrogen (to ensure the anaerobic condition of the equipment), seal the polymerization tube, heat at 145 ° C, and react for 12 hours to obtain the number average TPGS-b-(PLA-ran-PGA) copolymers (I) and (II) with a molecular weight of 4300.
[0049] TPGS-b-(PLA-ran-PGA) copolymer crude product (I) and (II) were dissolved in methylene chloride, methanol was added to make TPGS-b-(PLA-ran-PGA) copolymer precipitate, filtered, and The precipitate was vacuum-dried at 50° C. to obtain refined TPGS-b-(PLA-ran-PGA) copolymers (I) and (II) with a molecular weight of 4300.
[0050] The TPGS-b-(PLA-ran-PGA) copolymer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com