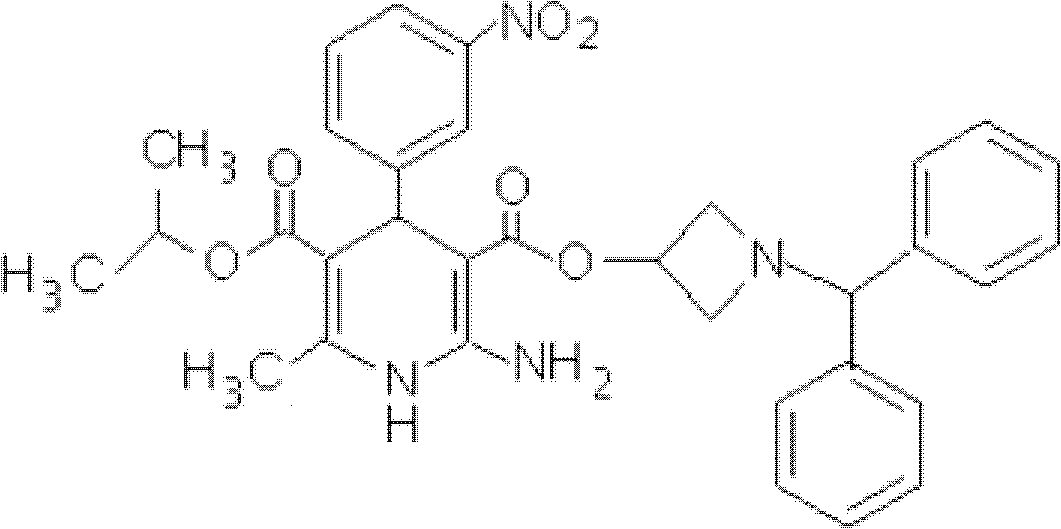
Azelnidipine quick-releasing drug preparation and preparation method
A zedipine and rapid technology, which is applied in the direction of drug combination, pharmaceutical formula, pill delivery, etc., can solve the problems of undisclosed dosage forms for rapid release of drugs, and achieve the effects of storage stability, loss reduction, and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
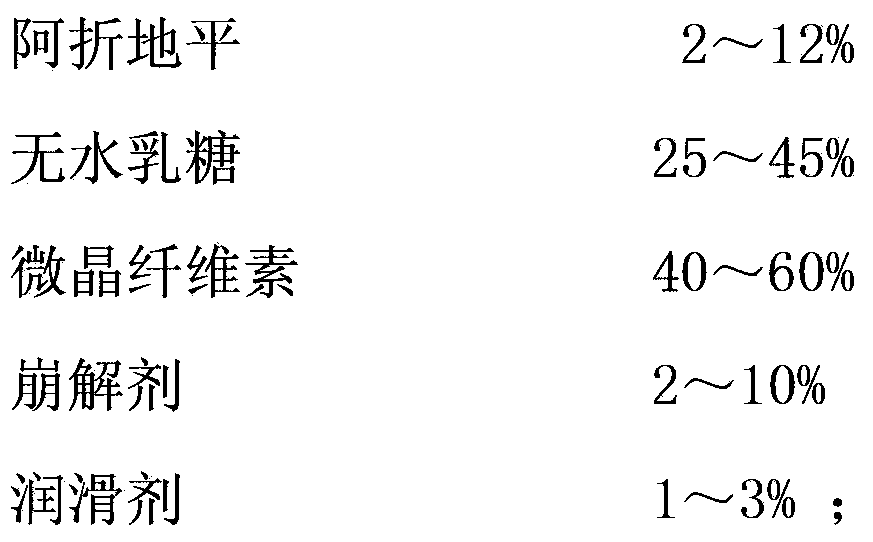
[0024] A kind of quick-release pharmaceutical preparation of Azedipine, comprises the component of following weight:
[0025]
[0026] The preparation method is as follows: uniformly mix azedipine, anhydrous lactose, microcrystalline cellulose, disintegrant and lubricant, pass through a 100-mesh sieve, repeat the mixing for 3 times, and then compress into tablets.
Embodiment 2
[0028] A kind of quick-release pharmaceutical preparation of Azedipine, comprises the component of following weight:
[0029]
[0030] The preparation method is the same as in Example 1.
Embodiment 3
[0032]
[0033] The preparation process is the same as in Example 1.
[0034] At the same time, the Azeldipine tablet prepared by the ordinary wet method was selected as Comparative Example 1, and the Azeldipine tablet prepared by the ordinary dry method was selected as Comparative Example 2.
[0035] Get the sample prepared by the present embodiment and comparative example, according to Chinese Pharmacopoeia 2010 edition two X C first method for dissolution rate assay, with 50% ethanol aqueous solution 500ml as solvent, rotating speed is 75 revs / min per minute, after 45 minutes Measuring the amount of drug dissolution, test 6 tablets under the same conditions, the results are as follows.
[0036] sample
[0037] It can be seen that the difference is significant, and the dissolution rate of the fast-release drug prepared by the present invention is 14.3% to 16.2% higher than that of samples prepared by common wet and dry granulation methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com