Method for preparing boron trifluoride through reacting fluosulfonic acid with boric acid
A technology of boron trifluoride and fluorosulfonic acid, which is applied in the direction of boron halide compounds, etc., can solve the problem of low production cost, and achieve the effects of low production cost, stable quality, and good use of cooling and heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
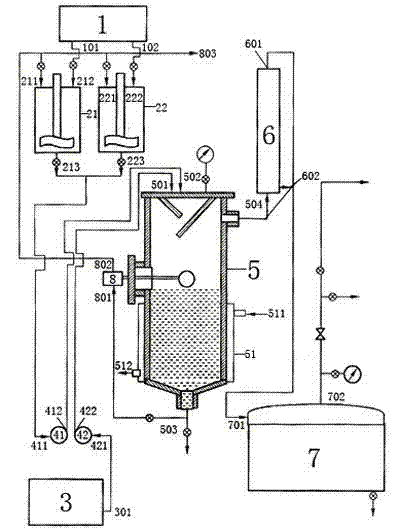
[0054] A method for preparing boron trifluoride by the reaction of fluorosulfonic acid and boric acid, which is to mix fluorosulfonic acid and boric acid and react to prepare boron trifluoride at a pressure of 0.2 MPA and a temperature of 87°C; wherein the boric acid and fluorosulfonic acid The weight fraction ratio is 1:2. The flow process described therein: adopt integrated continuous feeding, solid boric acid feeding tank 1 enters dissolving tank 21 and dissolving tank 22 through a feeder, and the boric acid after heating and melting in the dissolving tank passes into boric acid pump 41 and enters reactor 5, at this time The fluorosulfonic acid feeding tank 3 in the fluorosulfonic acid storage tank is also driven into the reactor 5 through the acid pump 42, and reacts at a pressure of 0.2MPA and a temperature of 87°C. The heat is provided by steam, and the reaction generates boron trifluoride gas through heat exchange. After the device 6 is cooled, it enters the collecting...
Embodiment 2
[0056] A method for preparing boron trifluoride by reacting fluorosulfonic acid with boric acid is characterized in that: it mixes fluorosulfonic acid and boric acid, and reacts to prepare boron trifluoride at a pressure of 0.4 MPA and a temperature of 93°C; wherein boric acid and boric acid The weight fraction ratio of fluorosulfonic acid is 1: 4, see figure 1 .
Embodiment 3
[0058] A method for preparing boron trifluoride by the reaction of fluorosulfonic acid and boric acid, which is to mix fluorosulfonic acid and boric acid and react to prepare boron trifluoride at a pressure of 0.2 MPA and a temperature of 87°C; wherein the boric acid and fluorosulfonic acid The weight fraction ratio is 1:2;
[0059] The flow process described therein: adopt integrated continuous feeding, solid boric acid feeding tank 1 enters dissolving tank 21 and dissolving tank 22 through a feeder, and the boric acid after heating and melting in the dissolving tank passes into boric acid pump 41 and enters reactor 5, at this time The fluorosulfonic acid feeding tank 3 in the fluorosulfonic acid storage tank is also driven into the reactor 5 through the acid pump 42, and reacts at a pressure of 0.2MPA and a temperature of 87°C. The heat is provided by steam, and the reaction generates boron trifluoride gas through heat exchange. After the device 6 is cooled, it enters the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com