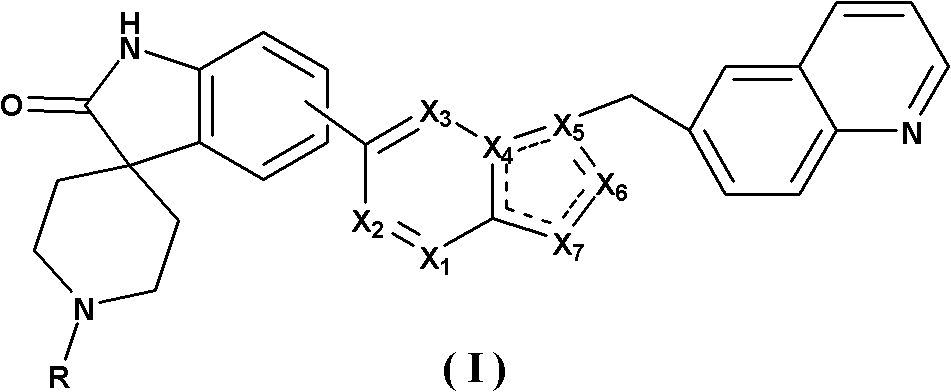
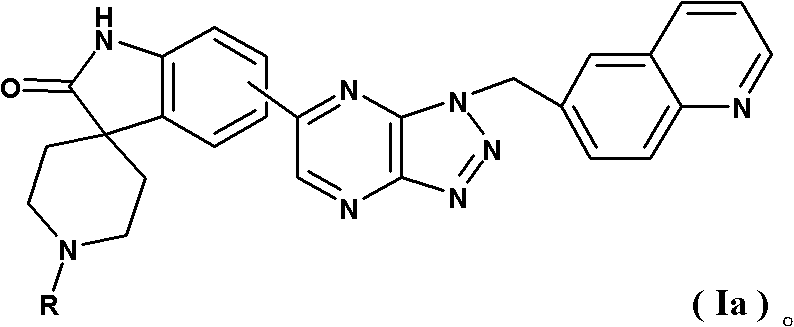
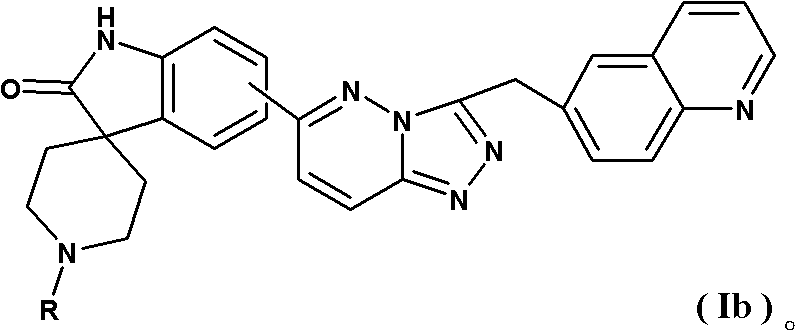
Spirotricyclic compound, its preparation method, and pharmaceutical composition containing it as well as application thereof
A kind of compound, technology of spiro tricyclic, applied in the field of spiro tricyclic compounds and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0289] 5-[1-(6-quinolinylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]spiro[indoline-3,4' Synthesis of -piperidin]-2-one
[0290]
[0291] Step 1: Synthesis of {2-oxyspiro[indoline-3,4'-piperidinyl]-5-yl}boronic acid pinacol ester
[0292]
[0293] Under nitrogen, add 5-bromospiro[indoline-3,4'-piperidin]-2-one (140.6mg or 0.5mmol), bipinacol borate (140mg or 0.55mmol) and potassium acetate (147mg or 1.5mmol) in DMSO solution (0.2ml) was added PdCl 2 (dppf).CH 2 Cl 2 (20.4 mg or 0.025 mmol), nitrogen was bubbled into the resulting solution for 2 minutes, and then stirred at 80° C. for 16 hours. LC-MS showed that the reaction was complete. After cooling to room temperature, 2 mL of water was added and extracted with DCM (5 mL was used for 3 extractions). Combine the organic phases with Na 2 SO 4 After drying and concentration, 162 mg of the target product was obtained (yield: 98.7%). Mass Spectrum m / z: 328.07 [M+H + ], 329.08[M+H + , 100%], 330.11 [M+H + ].
...
Embodiment 2
[0297] 6-[1-(6-quinolinylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]spiro[indoline-3,4' Synthesis of -piperidin]-2-one
[0298]
[0299] Step 1: Synthesis of {2-oxyspiro[indoline-3,4'-piperidinyl]-6-yl}boronic acid pinacol ester
[0300]
[0301] Referring to the method of Example 1, it was prepared from 6-bromospiro[indoline-3,4'-piperidin]-2-one, yield: 96.9%. Mass Spectrum m / z: 328.07 [M+H + ], 329.08[M+H + , 100%], 330.11 [M+H + ].
[0302] Step 2: 6-[1-(6-Quinolinylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]spiro[indoline-3 , Synthesis of 4'-piperidin]-2-one
[0303] Prepare with reference to the method of Example 1. Yield: 73%. Mass Spectrum m / z: 463.23 [M+H + ].
Embodiment 3
[0305]1'-Methyl-5-[1-(6-quinolylmethyl)-1H-[1,2,3]triazol[4,5-b]pyrazin-6-yl]spiro[indole Synthesis of phen-3,4'-piperidin]-2-one
[0306]
[0307] Step 1: Synthesis of 5-bromo-1'-methylspiro[indoline-3,4'-piperidin]-2-one
[0308]
[0309] 5-Bromospiro[indoline-3,4'-piperidin]-2-one (1.686g or 6mmol) was suspended in 15mL THF, cooled to -78°C, and 1M NaN (SiMe 3 ) 2 THF solution (30mL or 30mmol). After the addition was complete, it was stirred at -78°C for 30 minutes, and then solid 2-chloro-N-(2-chloroethyl)-N-methylethylamine hydrochloride (1155 g or 6 mmol) was added. Stirring was continued for 30 minutes after the addition was complete, then raised to room temperature and stirred for two days. TLC showed that the reaction was complete, and 10 mL of 4M hydrochloric acid aqueous solution was carefully added to the pink suspension, then adjusted to pH ≈ 9 with concentrated ammonia water, and extracted with DCM (3 times with 80 mL for each extraction). Combine the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com