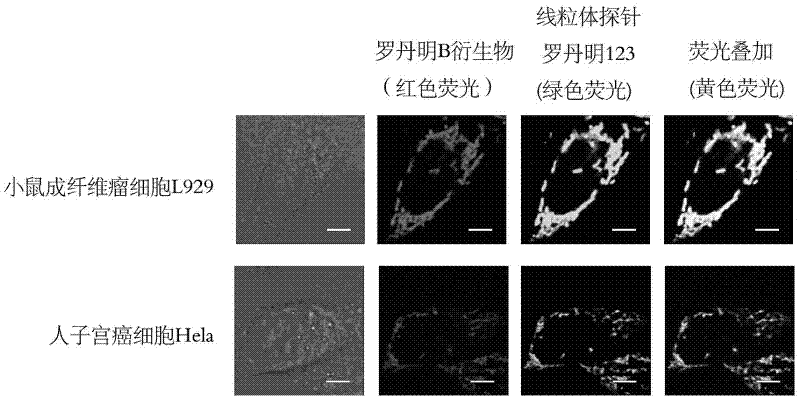
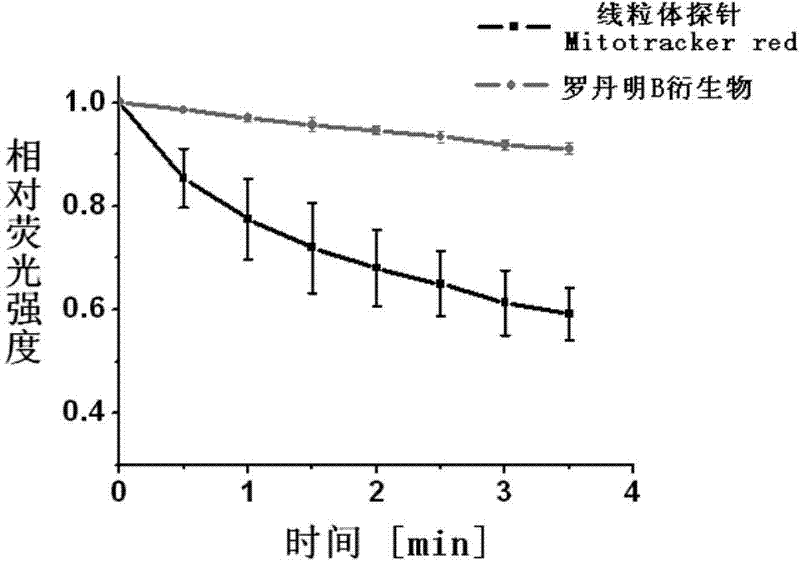
Application of rhodamine B derivative as cellular mitochondrion fluorescence coloring agent
A derivative and mitochondrial technology, which is applied in the field of cell mitochondrial fluorescent dyes, can solve the problems of short retention period and loss of probes, and achieve the effect of long retention time, high photostability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] 1) Rhodamine B (g), triphenylphosphine (g), hexachloroethane (g), tert-butoxypiperazine (g) were sequentially added to a round bottom containing 25ml triethylamine and 100ml dichloromethane In the flask, the electromagnetic stirring system was used. After reacting at room temperature for 12 hours, the initial product was separated and purified by silica gel column, and the initial product was separated and purified by silica gel column after being treated with trifluoroacetic acid. React the product with chloroacetic anhydride at room temperature for 12 hours, then separate and purify again to obtain Rhodamine B-methylchlorocarbonylpiperazine;
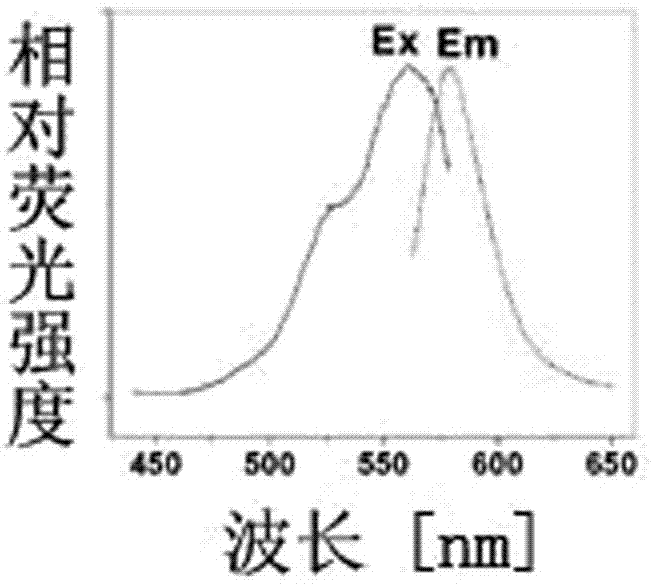
[0025] 2) Rhodamine B-methylchlorocarbonylpiperazine (1mg / ml) was dissolved in methanol, and the excitation and emission fluorescence spectra were detected. According to the attached figure 1 The excitation and emission fluorescence spectrograms of Rhodamine B-methylchlorocarbonylpiperazine shown can be found to have a maximum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com