Isoproterenol hydrochloride injection and preparation process thereof
A technology of isoproterenol hydrochloride and injection, which is applied in drug delivery, cardiovascular system diseases, drug combination, etc., can solve the problems of high prescription cost and unachievable preparation method, and achieve the effect of low cost of prescription and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Isoproterenol hydrochloride injection contains the following ingredients:
[0047] Each 10000ml injection contains,
[0048] Isoproterenol Hydrochloride 5g
[0050] Sodium metabisulfite 10g
[0051] Edetate Disodium 3g
[0052] Add water for injection to 10000ml.
[0053] Its preparation process is:
[0054] (1) Add 90% of the total volume of water for injection in the preparation container, cool to below 30°C, and pass through CO 2 15 minutes to make it saturated;
[0055] (2) Dissolve edetate disodium in hot water for injection at 80°C to 85°C, and set aside;
[0056] (3) Start the agitator, add sodium chloride, sodium metabisulfite, isoproterenol hydrochloride and the disodium edetate solution obtained in step (2) into the water for injection obtained in step (1) in sequence, and stir for 5 minutes to dissolve ;
[0057] (4) Adjust the pH value of the solution obtained in step (3) with 10% hydrochloric acid or 5% ...
Embodiment 2
[0068] Isoproterenol hydrochloride injection contains the following ingredients:
[0069] Each 10000ml injection contains,
[0070] Isoproterenol Hydrochloride 5g
[0071] Sodium chloride 60g
[0072] Edetate Disodium 1g
[0073] Add water for injection to 10000ml.
[0074] The preparation technology of described isoprenaline hydrochloride injection comprises the following steps:
[0075] (1) Add 90% of the total volume of water for injection in the preparation container, cool to below 30°C, and pass through CO 2 15 minutes to make it saturated;
[0076] (2) Dissolve edetate disodium in hot water for injection at 80°C to 85°C, and set aside;
[0077] (3) Start the agitator, add sodium chloride, isoproterenol hydrochloride and edetate disodium solution obtained in step (2) into the water for injection obtained in step (1) in sequence, and stir for 5 minutes to dissolve;
[0078] (4) Adjust the pH value of the solution obtained in step (3) with 10% hydrochlori...
Embodiment 3
[0087] Isoproterenol hydrochloride injection contains the following ingredients:
[0088] Each 10000ml injection contains,
[0089] Isoproterenol Hydrochloride 5g
[0090] Sodium chloride 85g
[0091] Edetate Disodium 3g
[0092] Add water for injection to 10000ml.
[0093] Its preparation technology is identical with embodiment 2.
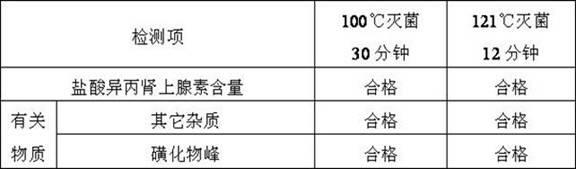
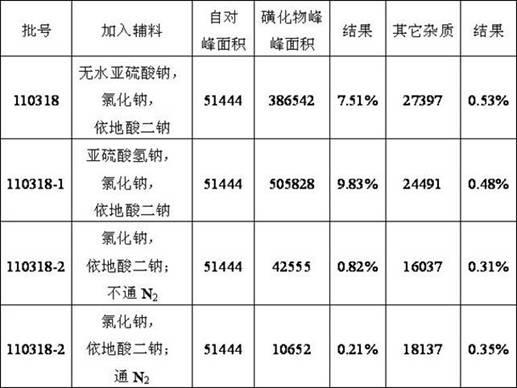
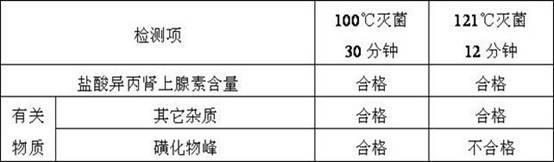
[0094] The obtained isoprenaline hydrochloride injection is carried out quality inspection, and its result is as follows:
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com