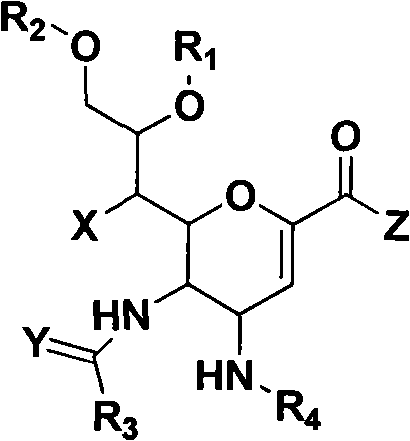
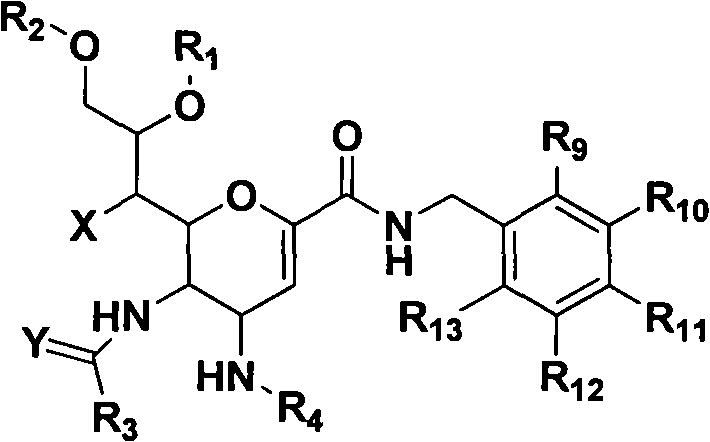
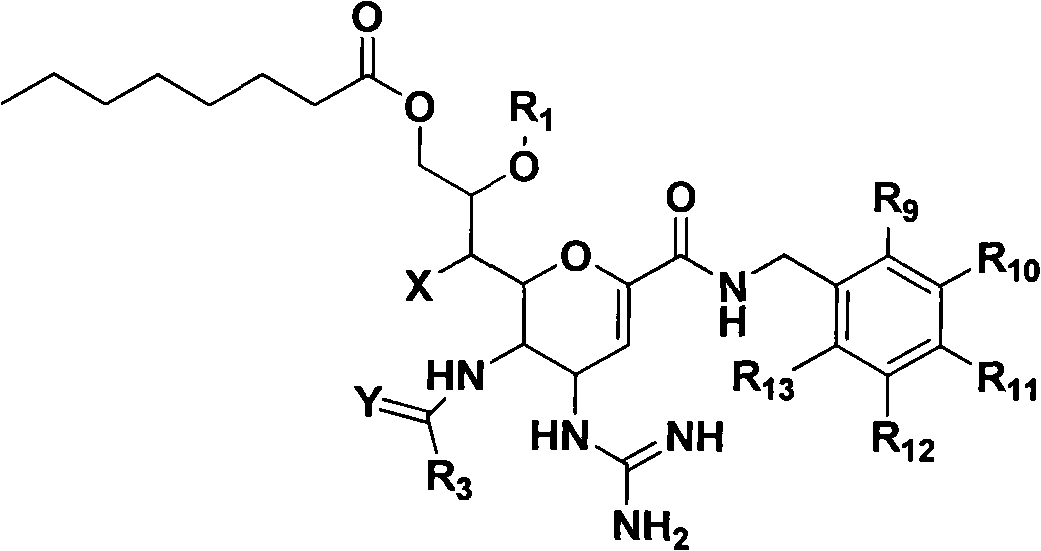
Novel sialic acid derivant, preparation method thereof, drug composite comprising the same and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problem of low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] (4S, 5R, 6R)-N-benzyl-4-guanidino-5-acetamido-6[(1R,2R)-1,2,3-trihydroxypropyl]-5,6-dihydro- Preparation of 4H-pyran-2-carboxamide (10a-1)
[0228] 1. Cesium 5α-acetylamino-2α, 4β-dihydroxy-6β-D-glyceryl-tetrahydro-2H-pyran-2-carboxylate (1a-1)
[0229]
[0230] Dissolve N-acetylneuraminic acid (NANA, 10g, 32.4mmol) in 10ml of water, add cesium carbonate (5g) to adjust the pH value to neutral (pH7-8), evaporate the solvent under reduced pressure, and pump dry to obtain glass fragments like solid 1a-1;
[0231] 2. Benzyl 5α-acetylamino-2α, 4β-dihydroxy-6β-D-glyceryl-tetrahydro-2H-pyran-2-carboxylate (2a)
[0232]
[0233] Add DMF (15ml) and benzyl bromide (6ml) to 1a-1, stir overnight at room temperature, the reaction solution slowly has a lot of white solids precipitate out, filter with suction, add dichloromethane (500ml) while the filtrate is stirred, white solids precipitate out, Suction filtration, washing with dichloromethane, and air-drying in a cool plac...
Embodiment 2
[0259] (4S, 5R, 6R)-N-[(4-methoxyphenyl)methyl]-4-guanidino-5-acetamido-6[(1R,2R)-1,2,3-trihydroxy Preparation of Propyl]-5,6-dihydro-4H-pyran-2-carboxamide (10a-2)
[0260]Benzylamine is replaced by 4-methoxybenzylamine, and the remaining raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product (4S, 5R, 6R)-N-[(4-methoxyphenyl)methyl base]-4-guanidino-5-acetylamino-6[(1R,2R)-1,2,3-trihydroxypropyl]-5,6-dihydro-4H-pyran-2-carboxamide. 1 H-NMR (CD 3 OD, 300MHz): δ2.01(s, 3H), 3.34(s, 2H), 3.65-3.78(m, 2H), 3.77(s, 3H), 4.16-4.22(m, 2H), 4.36-4.42( m, 3H), 5.72(s, 1H), 6.86(d, 8.4Hz, 2H), 7.22(d, 8.4Hz, 2H); LC-MS m / z 452[M+H] + ;
Embodiment 3
[0262] (4S, 5R, 6R)-N-[(3-methoxyphenyl)methyl]-4-guanidino-5-acetamido-6[(1R,2R)-1,2,3-trihydroxy Propyl]-5,6-dihydro-4H-pyran-2-carboxamide (10a-3)
[0263] Benzylamine is replaced by 3-methoxybenzylamine, and the remaining raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product (4S, 5R, 6R)-N-[(3-methoxyphenyl)methyl Base]-4-guanidino-5-acetylamino-6[(1R,2R)-1,2,3-trihydroxypropyl]-5,6-dihydro-4H-pyran-2-carboxamide; 1 H-NMR (CD3OD, 300MHz): δ2.01(s, 3H), 3.66-3.70(m, 2H), 3.77-3.82(m, 4H), 4.18-4.24(m, 2H), 4.41-4.49(m , 5H), 5.74(d, 2.4Hz, 1H), 6.81(d, 9.6Hz, 1H), 6.87(d, 7.5Hz, 1H), 7.22(t, 8.4Hz, 1H), 7.30(s, 1H) ;LC-MS m / z 452[M+H] + ;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com