High-stability Recomb Protein A having antibody binding capacity and preparation thereof
A technology of binding ability and recombinant protein, which is applied in the biological field and can solve problems such as protein A instability and affecting use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, gene sequence and corresponding protein sequence
[0091] The SPA (32-327) gene sequence is as follows (SEQ ID NO: 1, 891bp):
[0092] CCTGCTGCAAATGCTGCGCAACACGATGAAGCTCAACAAAATGCTTTTTATCAAGTTTTAAATATGCCTAACTTAAATGCTGATCGACGCAATGGTTTTATCCAAAGCCTTAAAGATGATCCAAGCCAAAGTGCTAACGTTTTAGGTGAAGCTCAAAAACTTAATGACTCTCAAGCTCCAAAAGCTGATGCGCAACAAAATAACTTCAACAAAGATCAACAAAGCGCCTTCTATGAAATTTTGAACATGCCTAACTTAAACGAAGCGCAACGCAATGGTTTCATTCAAAGTCTTAAAGACGATCCAAGCCAAAGCACTAACGTTTTAGGTGAAGCTAAAAAATTAAACGAATCTCAAGCACCGAAAGCTGACAACAATTTCAACAAAGAACAACAAAATGCTTTCTATGAAATCTTGAACATGCCTAACTTGAACGAAGAACAACGCAATGGTTTCATCCAAAGCTTAAAAGATGACCCAAGTCAAAGTGCTAACCTATTGTCAGAAGCTAAAAAGTTAAATGAATCTCAAGCACCGAAAGCGGATAACAAATTCAACAAAGAACAACAAAATGCTTTCTATGAAATCTTACATTTACCTAACTTAAACGAAGAACAACGCAATGGTTTCATCCAAAGCTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAGCTAAAAAGCTAAATGATGCACAAGCACCAAAAGCTGACAACAAATTCAACAAAGAACAACAAAATGCTTTCTATGAAATTTTACATTTACCTAACTTAACTGAAGAACAACGTAACGGCTTCATCCAAAGCCTTAAAGACGATCCTTCAGTGAGC...
Embodiment 2
[0143] Cloning and expression identification of embodiment 2, SPA (FL) full-length gene and SPA (32-327) gene
[0144] 1. PCR amplification to obtain truncated SPA (32-327) and full-length SPA (FL)
[0145] Staphylococcus aureus was cultivated, and its genomic DNA was extracted by conventional methods.
[0146] The following amplification primers were designed to amplify the gene encoding the truncated SPA (32-327):
[0147] Forward: CGCCATATGCCTGCTGCAAATGCTGCGC (SEQ ID NO: 7)
[0148] Reverse: CCGCTCGAGTTATTTTGGTGCTTGAGC (SEQ ID NO: 8)
[0149] The following amplification primers were designed to amplify the gene encoding full-length SPA (FL):
[0150] Forward: CGCCATATGTTGAAAAAGAAAAACATTTATTC (SEQ ID NO: 9)
[0151] Reverse: CCGCTCGAGTTATAGTTCGCGACGACG (SEQ ID NO: 10)
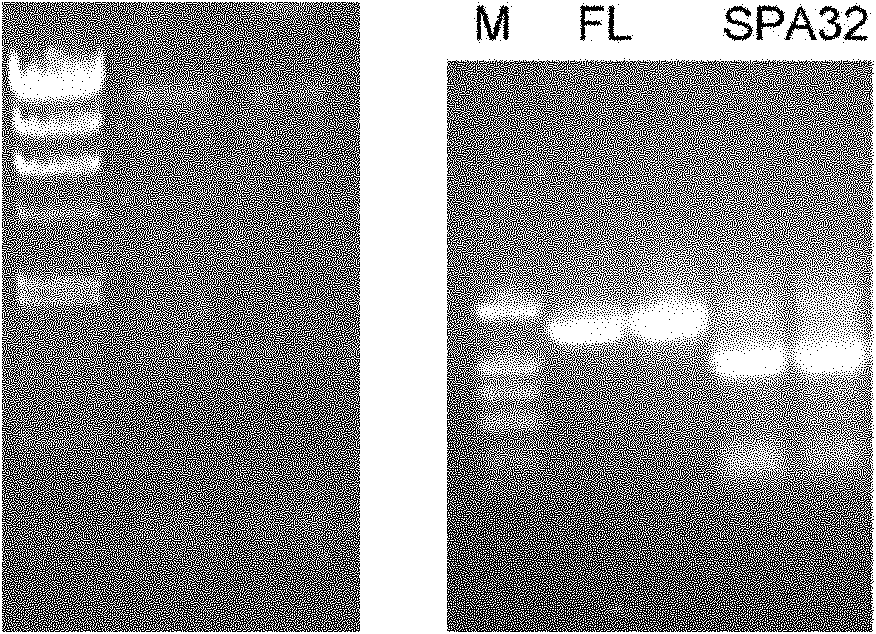
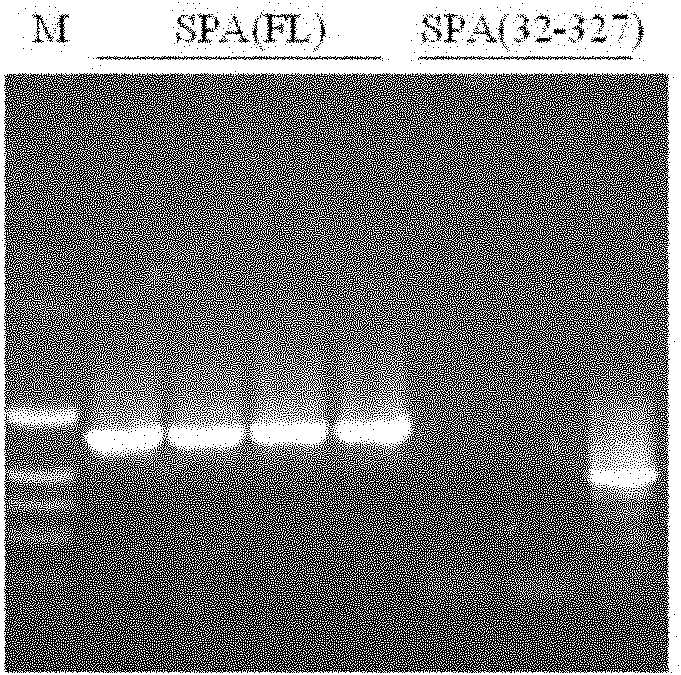
[0152] The above primers were used to amplify the full-length SPA (FL) and the truncated SPA (32-327) respectively with the genomic DNA of Staphylococcus aureus as a template. The results of electrophor...
Embodiment 3
[0155] Embodiment 3, expression optimization of SPA (32-327)
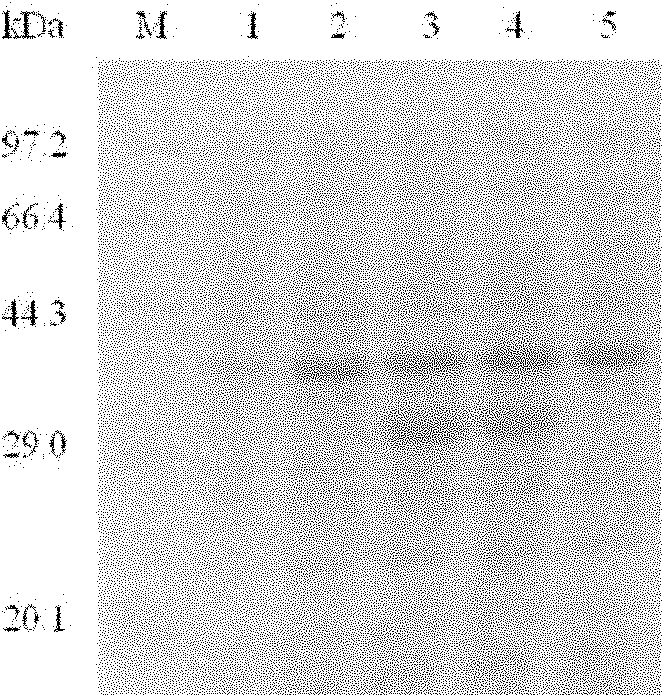
[0156] Please provide the specific method for culturing recombinant BL21(DE3) cells: Pick the single clone colony on the transformation plate, inoculate it into 30mL LB culture medium (containing 100μg / mL Amp), and culture it overnight on a shaker at 37°C (10-12h ), transfer it to a secondary bottle with 2% inoculum size, and continue to cultivate. When the bacterial density reached OD600 0.5-0.6, add IPTG at a final concentration of 0.5mmol / L at 37°C and 12°C for induction for 4 hours, collect the bacterial cells by centrifugation at 10,000rpm, discard the supernatant, ultrasonically disrupt the bacterial cells, and centrifuge. The supernatant and precipitate were identified by electrophoresis.
[0157] Based on the above methods, the effects of different temperatures on the expression of SPA(32-327) and the effects of different IPTG concentrations on the expression of SPA(32-327) were investigated.
[0158] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com